1. Background
Fever is defined as an increase in the core body temperature ≥ 38.3°C (1). The incidence of fever ranges between 28% and 75% in critically ill patients (2, 3). Fever depends on a complex physiologic response to infectious agents and other conditions such as pulmonary embolism, gastrointestinal bleeding, drug reactions, trauma, surgery, and etc. (2, 4, 5). During a febrile episode, an increase of oxygen consumption and energy expenditure occurs (6). Subsequent increases in oxygen consumption, respiratory quotient, and cardiac output add a considerable burden to febrile patients who might be unable to compensate for the increased metabolic demand (7, 8). With the increasing of oxygen demand in cell levels during fever, easily separated bunding oxygen on Hb in the cells occur (6, 9). Based on this information, blood gas values are directly affected by fever (10, 11).
Fever is a manifestation of cytokine release in response to a variety of stimuli. Cytokines may be released in response to exogenous pyrogens such as microorganisms (12). Cytokines stimulate the release of prostaglandins from the anterior hypothalamus and the prostaglandins are then responsible for acting to reset the thermoregulatory center, thereby, causing a rise in the temperature set point that manifests as a fever (13). Besides evaluating the febrile patient and initiating a workup based on the clinical evaluation, it is common for the patients to receive either pharmacologic or mechanical antipyretic therapy (14, 15). Clinicians have controlled fevers by using external cooling methods and antipyretics (APs) (16, 17). Studies showed that the use of APs to diminish fevers correlates with negatively affecting patient outcomes (18). APs act mainly by the inhibation of prostaglandin synthesis or decrease prostaglandin E2 levels during fever (5, 19). Furthermore, APs have some adverse effects such as hypotension, renal, and hepatic toxicity (20). In the study of Vera et al. the effects of different APs on hemodynamic parameters decrease in systolic blood pressure were observed (16). Although, non-pharmacological and pharmacological methods are used to reduce core BT in febrile patients, little research related to the effects of these methods on BT and hemodynamic parameters have been performed (21-23). In the present study we aimed to determine the effects of peripheral cold application (PCA) and APs on BT and heamodynamic parameters (systolic/diastolic and mean arterial blood pressure, pulse rate, repiratory rate and arterial O2 saturation) in febrile ICU adult patients diagnosed with hospital-acquired infections (HAIs).
2. Methods
This retrospective study was conducted by evaluating the documents of the patients diagnosed with HAIs in the internal-surgical ICUs at a university hospital in the west of Turkey between 2014 - 2015. It was determined that in total, 9547 patients stayed in the adult and pediatrics ICUs, and 448 of these patients were diagnosed with a HAIs. A total of 346 of the 448 patients that stayed in the adults ICUs had HAIs and related documents were evaluated. In order to be able to carry out the study, written permissions from Adnan Menderes University Non-Invasive Ethics Committee, Chief Physician of Adnan Menderes University Hospital, and the Head of the Department of Infectious Diseases were received. One hour before, during, and one hour after PCA or APs interventions related to febrile episodes, BT, SBD, DBS, PR, RR, and aO2Sat values were recorded. For statistical analysis, SPSS version 17 (SPSS Inc., Chicago, IL) was used. Shapiro Wilk test was used to check the normality of distribution of continuous variables. As appropriate, data are presented as percent and mean (SD). To compare mean values, paired t test and one-way ANOVA with repeated measures test were used. Statistical significance was set at P < 0.05, as appropriate.
3. Results
The mean age of the patients was 67.91 ± 14.76, mean length of stay in the ICU was 27.02 ± 25.68 days. A total of 46% and 31.8% of the patients had bacteremia and VIP, respectively; 16.8% of the causative microorganisms were Acinetobacter spp.
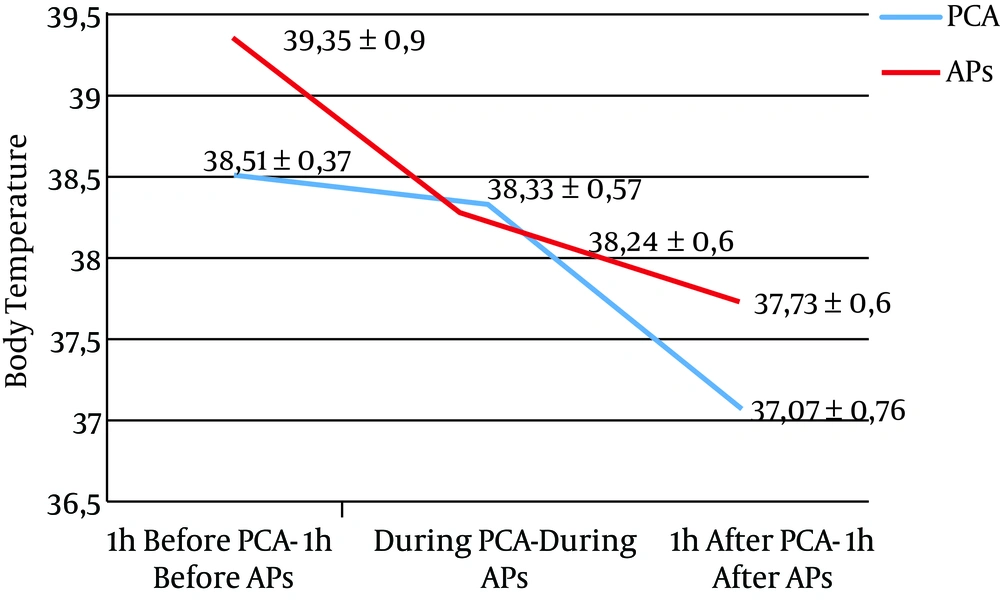
A decrease of 0.17°C (P = 0.004) and 0.44°C (P < 0.001) of mean BT was observed during and one hour after PCA compared with one hour before, respectively. A decrease of 0.16°C (P = 0.004) and 0.65°C (P < 0.001) was observed during and one hour after APs compared with one hour before, respectively (Figure 1).
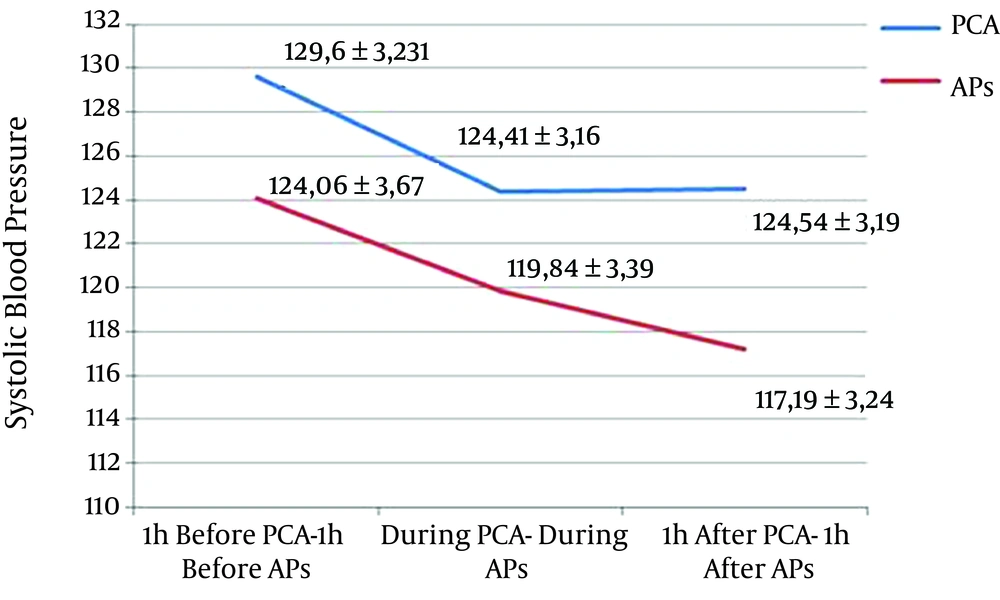
A decrease of 5.19 mmHg (P = 0.002) and 5.06 mmHg (P = 0.009) of mean SBP was observed during and one hour after PCA compared with one hour before, respectively. A decrease of 3.55 mmHg (P = 0.121) and 5.73 mmHg (P = 0.010) was observed during and one hour after AP compared with one hour before, respectively (Figure 2).
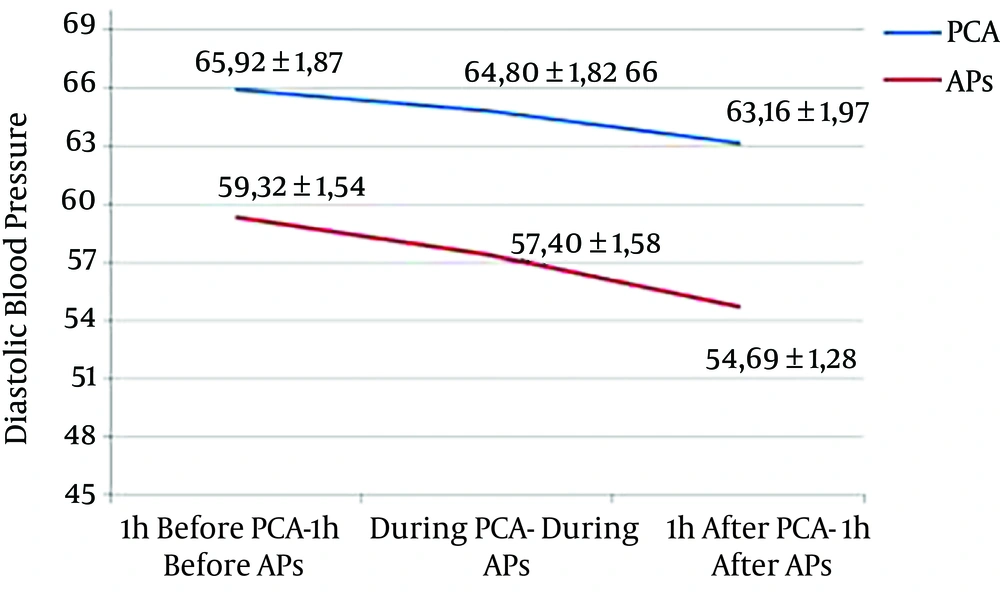
A decrease of 1.18 mmHg (P = 0.249) and 2.76 mmHg (P = 0.049) of mean DBP was observed during and one hour after PCA compared with one hour before, respectively. A decrease of 2.69 mmHg (P = 0.039) and 5.33 mmHg (P < 0.001) was observed during and one hour after AP compared with one hour before, respectively (Figure 3).
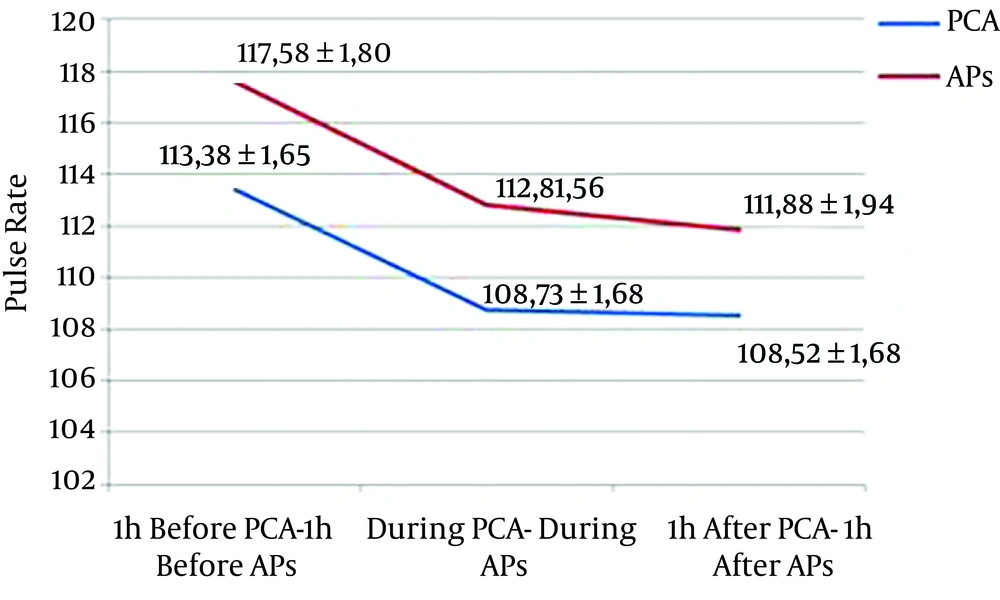
A decrease of 3.85 beats/min (P = 0.003) and 5.20 beats/min (P < 0.001) of mean PR was observed during and one hour after PCA compared with one hour before, respectively. A decrease of 4.63 beats/min (P < 0.001) and 5.69 beats/min (P < 0.001) was observed during and one hour after AP compare with one hour before, respectively (Figure 4).
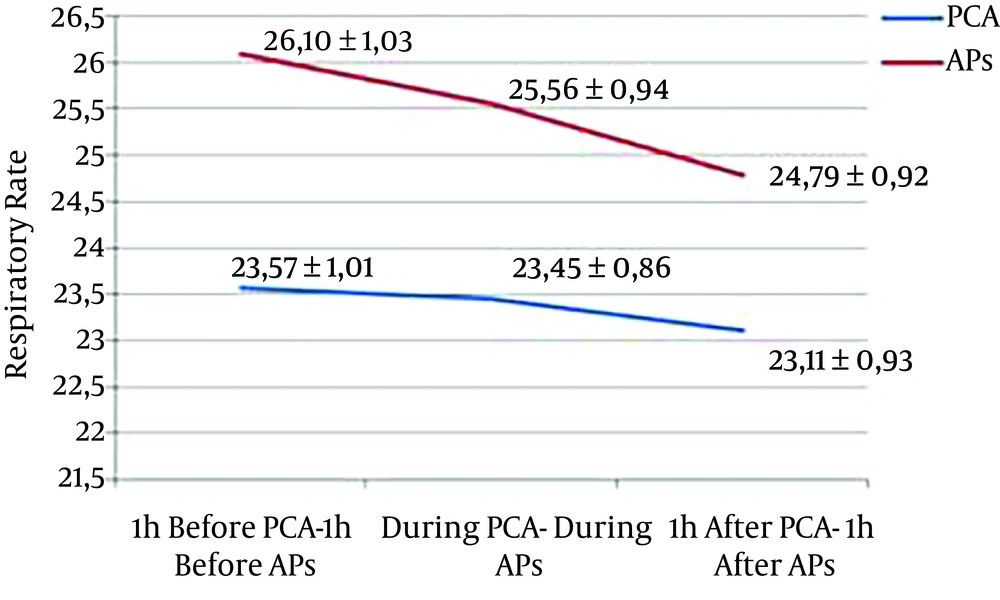
A decrease of 0.11 breaths/min (P = 0.850) and 0.46 breaths/min (P = 0.585) of mean RR was observed during and one hour after PCA compared with one hour before, respectively. A decrease of 0.10 breaths/min (P = 0.887) and 1.12 breaths/min (P = 0.209) was observed during and one hour after AP compared with one hour before, respectively (Figure 5).
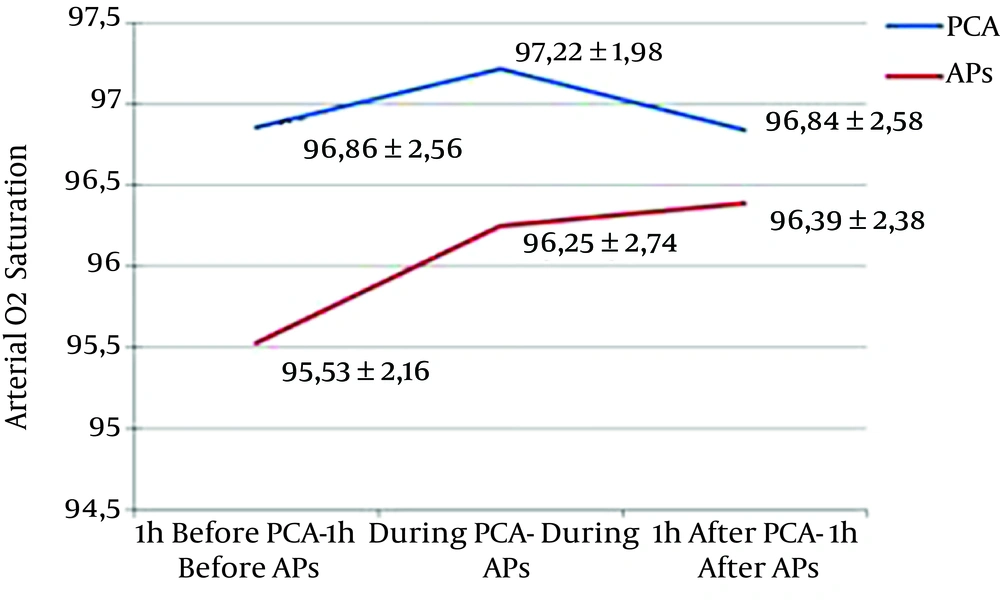
An increase of 0.37% (P = 0.134) and a decrease of 0.02% (P = 931) of mean aO2Sat was observed during and one hour after PCA compared with one hour before, respectively. An increase of 0.68% (P = 0.139) and 0.86 % (P = 0.020) was observed during and one hour after AP compared with one hour before, respectively (Figure 6).
Comparing BT and hemodynamic parameters over time, from one hour before, during, and one hour after, PCA, BT, DBP, PR, and aO2Sat parameters did significantly change (P < 0.05), while SBP and RR parameters did not significantly changes (P > 0.05). Comparing BT and hemodynamic parameters over time, from one hour before, during, and one hour after APs, BT, RR, and aO2Sat parameters did significantly change (P < 0.05), while SBP, DBP, and PR parameters did not significantly changes (P > 0.05) (Table 1).
| Variables | 1 Hour Before PCA | During PCA | 1 Hour After PCA | P Value | 1 Hour Before APs | During APs | 1 Hour After APs | P Value |
|---|---|---|---|---|---|---|---|---|
| BT,°C | 38.51 ± 0.37 | 38.33 ± 0.57 | 38.07 ± 0.76 | < 0.001a | 39.35 ± 0.90 | 38.25 ± 0.60 | 37.73 ± 0.60 | < 0.001a |
| SBP, mmHg | 129.61 ± 3.23 | 124.41 ± 3.16 | 124.54 ± 3.19 | 0.250 | 124.06 ± 3.67 | 119.84 ± 3.39 | 117.19 ± 3.24 | 0.865 |
| DBP, mmHg | 65.92 ± 1.87 | 64.80 ± 1.82 | 63.16 ± 1.97 | 0.002a | 59.32 ± 1.54 | 57.40 ± 1.58 | 54.69 ± 1.28 | 0.492 |
| PR, beats/min | 113.38 ± 1.65 | 108.73 ± 1.68 | 108.52 ± 1.68 | 0.003a | 117.58 ± 1.80 | 112.80 ± 1.56 | 111.88 ± 1.94 | 0.700 |
| RR, breaths/min | 23.57 ± 1.01 | 23.45 ± 0.86 | 23.11 ± 0.93 | 0.080 | 26.10 ± 1.03 | 25.56 ± 0.94 | 24.79 ± 0.92 | < 0.001a |
| O2Sat, % | 96.86 ± 2.56 | 97.22 ± 1.98 | 96.84 ± 2.58 | 0.004a | 95.53 ± 2.16 | 96.25 ± 2.74 | 96.39 ± 2.38 | < 0.001a |
a Significantly change.
4. Discussion
In the present study, the incidence of the HIAs was 4.16%, and 46% of the patients had a bacteremia infection, and the most causative microorganism was Acinetobacter spp. In the O'Shea et al. (24) study 28.8% of the ICU patients developed an infection and the responsible organisms included Pseudomonas, Acinetobacter, E. coli, ect. (18). In the Erman et al. (25) study 6.2% of neurosurgery patients developed an infection and the predominantly isolated microorganisms were SA, Acinetobacter baumanii, and Staphylococcus epidermidis (19). In the Sturm (26) study the majority of microorganisms in craniotomy wounds were gram-positive (20). In the Celik et al. study Acinetobacter and Pseudomonas were the most common causes of nosocomial infections in ICU patients (4). Most studies stated that the most frequent HIAs in ICU patients were urinary tract infections (24-27). Erbay et al. (28), Meric et al. (29), Akhtar, (30) Baghaei et al. (31), Pradhan et al. (32) and Khan et al. (33) stated that the most frequent HIAs in ICU patients were respiratory tract infections (28-30, 34-36). In the study of Dereli et al. (37), the most frequent HIAs were bloodstream, soft tissue, and skin infections (31). In the study of Farzianpour et al. (36) and Amazian et al. (38) E. coli was the most isolated microorganism in HIAs (27, 32). In the study of de Oliveira et al. (34), Pradhan et al. (32), Dereli et al. (37), Alatrouny and Luna, Acinetobacter baumanii was the most isolated microorganism (25, 29, 31, 33, 37). In the Erbay et al. (28), Akhtar, Baghaei et al. (31) and Khan et al. (33) study, Pseudomonas aeruginosa, and in the Kolgeliler study, MRSA were the most isolated microorganisms (28, 30, 34, 36, 38).
Fever is a complex physiologic response triggered by infectious or aseptic stimuli. Elevations in body temperature occur when concentrations of prostaglandin E2 increase within certain areas of the brain (22). The induction and maintenance of fever during infection involves the tightly coordinated interplay between the innate immune system and neuronal circuitry within the central and peripheral nervous systems (23). At the persent study, BT, DBS, PR, and aO2Sat changes one hour before, during, an done hour after PCA were significantly different, and BT, RR, and aO2Sat changes one hour before, during, and one hour after APs were significantly different. More decrease in SBP and DBP were observed between during and one hour after APs compared with PCA.
In the studies of Kiekkas et al. (6) and Asgar Pour and Yavuz (9) fever episodes were followed by changes in hemodynamic parameters. In the present study, BT and hemodynamic parameters changes were higher in AP compared with PCA. In the study by Polderman et al. (39), on neurology, febrile patients, to avoid myocardial and neurological damage mild to moderate hypotermia, applied cold water, drafted a blanket, and applied cold normal salin infusion. The MAP increased 15 mmHg, this parameter was seen higher in patients without hemodynamic instability. The study results showed that hypothermia is achieved safely and rapidly with cold water, drafted blanket, and cold normal salin infusion together (39). Cormio and Citero study results to assess two different APs effects on MAP and ICP and evaluation their efficacy in fever control on patients with brain injury showed that the CPP and MAP were found high in the diclofenac applied patient group (40). In the study by Hata et al. results reducing the core BT effects oxygen consumption in acute brain injury (7).
In conclusion, as the rate of the HAIs is the most important indicator in the quality of care, it becomes important for the ICU clinicians to use their roles as a caregiver and as an educator in the prevention of the HAIs. Moreover, knowledge regarding the effects of APs on BT and hemodynamic parameters will be of a benefit to clinicians in terms of quality of care in ICU patients.