1. Background
According to statistical data, prostate cancer (PC) is the most prevalent tumor in men worldwide and is the second leading cause of cancer deaths among men in the United States. The number of new prostate cancer cases in the 2011 - 2015 period, by the National Cancer Institute, USA, was 112.6 per 100000 men per year and the number of deaths during this period was 19.5 per 100000 men per year (1, 2).
Prostate cancer cells first proliferate within the local tissue and then metastasize, which is mainly metastasis to the bone. The bone metastases may lead to clinical problems, including severe bone pain, impaired mobility, spinal cord compression, pathologic fractures, and bone marrow aplasia and hypercalcemia (3). Cell motility is a critical determinant of prostate cancer progress and metastasis.
Lysophosphatidic acid (LPA) is an important biofilm phospholipid that has been founded in many pathological and physiological biological fluids, such as plasma, serum, seminal fluid, and tears (4). In addition, LPA is made in numerous of cells, such as erythrocytes, endometrial cells, neurons, and ovarian cells.
The LPA has at least six receptors belonging to the 7- transmembrane G protein-coupled receptors (GPCRs) family that is named LPA1-6. The functional roles of LPA are controlled by extracellular signaling through these receptors. The LPA signaling mechanism starts by attaching to its receptors and activating heterotrimeric G proteins, such as G12/13, Gi/o, Gs, and Gq. This leads to the activation of secondary messengers, causing its molecular functions (5, 6).
GPCRs are the largest family of membrane proteins involved in intracellular messaging that act as the receptors for ions, neurotransmitters, hormones, photons, and other stimuli. Therefore, GPCRs control many physiological functions, including the release of hormones and enzymes, neuronal transmissions, smooth muscle contractions, immune responses, and regulation of blood pressure in the body (7).
It’s been proved in the previous studies that LPA increases the migration of prostate cancer cells, including primary prostatic cancer cells and prostate cancer cell lines such as DU145, PC3, and LNCaP cells (8, 9). On the other hand, it has been reported that LPA1, LPA2, and LPA3 (LPA receptors) have been expressed in prostate cancer cells (10). These receptors play an essential role in cellular responses; particularly, LPA1 has been shown that play an essential role in LPA-induced migration of prostate cancer cells (LNCaP and PC3 cells). However, so far, the signaling mechanism of this process has not been determined.
In addition, the expression of the LPA receptor or the plasma LPA level in patients with cancers is significantly higher than that of healthy people including ovarian cancer, acute or chronic myeloid leukemia, or colorectal cancer. Today, LPA is a potential biomarker for ovarian cancer (11). Other studies have shown that in acute myeloid leukemia, LPA production increases via Autotaxin (ATX), which results in the development of features that involve in the pathogenesis of this cancer including, reduction of the blood cells proliferation control and increasing their migration after bleeding (12).
Multiple pathological and clinical features have been considered as potential prognostic factors in PC: I. Gleason grade (assessing the grade of prostate cancer differentiation by prostate biopsy): The Gleason score 7, in most tumors (75% - 80%), indicates moderate differentiation of them. II. Prostate-specific antigen (PSA), in men under the age of 40 years with PSA more than 1 ng/mL, increases the risk developing prostate cancer and should be monitored periodically. III. Cancer stage at diagnosis: It has been determined that 70% - 80% of prostate cancers proliferate locally (13).
However, the sensitivity of the diagnosis is not definite, and sometimes the patients have normal PSA. The prostatic antigen test may also be increased in cases of prostate inflammation, benign prostatic enlargement, and also by colonoscopy. Therefore, for the definitive diagnosis of the disease, tissue sampling, and pathological examination is necessary (14, 15). Also, many people who do not have prostate cancer must tolerate the pain caused by sampling. Therefore, the use of other markers along with these tests can be effective in increasing the specificity and reliability of diagnostic tests.
2. Objectives
Since no studies have ever reported LPA levels in the serum of patients with prostate cancer, and on the other hand, because of the high level of LPA in the cancers mentioned earlier, this study was designed to evaluate lysophosphatidic acid (LPA) levels in the serum of patients with prostate cancer.
3. Methods
A two-group, non-randomized study was designed to evaluate the amount of lysophosphatidic acid in the serum of patients with prostate cancer. All participants will provide written informed consent before sampling, and the study protocol has been approved by the Ethics Committee of the AJA University of Medical Sciences, Tehran, Iran (No. 995752). To observe the principle of trust in the writing of the article, test results were published without names and identities.
After collecting blood samples from 20 healthy individuals and 20 patients with prostate cancer who referred to the Shariati Hospital in Tehran, serum was separated from each sample. For this purpose, 5 mL of fasting venous blood was taken from the participants. Blood samples were centrifuged at 3000 rpm for 10 minutes to separate serum from them. Collected serum samples were kept at -80°C in the freezer until tests were performed. At first, demographic information in regards to the patients, including weight, height, the result of the Gleason score, the result of the PSA test, and the history of radiation or surgical treatment were recorded. Then, the body mass index (BMI) and age of both groups were evaluated. The body mass index is the metric presently in use for describing anthropometric height/weight characteristics in adults and for categorizing them into groups. BMI is a person’s weight in kilograms (kg) divided by his or her height in meters squared. The National Institutes of Health (NIH) now defines normal weight, overweight, and obesity according to BMI rather than the traditional height/weight charts. Overweight is a BMI of 27.3 or more for women and 27.8 or more for men. Obesity is a BMI of 30 or more for either sex (about 30 pounds overweight).
In this study, the inclusion criteria included male gender, no disturbance in lipid metabolism, such as diabetes-related illness, and had no history of taking interfering drugs with lipid and carbohydrate metabolism.
To measure LPA in serum samples, the enzyme-linked immunosorbent assay (ELISA) technique (ELISA) was used. This technique was performed using the ELISA Kit manufactured by the ZellBio Company in Germany under the catalog number ZB-3406-H9648 and by following the manufacturer’s instructions.
SPSS V. 24 software (SPSS Inc., Chicago, IL, USA), Pearson correlation test, and unpaired student t-test were used for statistical analysis of the data. All data were calculated as the mean ± standard error of the mean (SEM). The receiver operating characteristic (ROC) was used to calculate the cut-off point and P < 0.05 was reflected as statistically significant.
4. Results
A comparison of the age of the participants in the two groups showed no significant differences between the mean age of prostate cancer and the control group (respectively 55.3 ± 2.3 and 53.3 ± 3.3 years) (P = 0.621).
The mean BMI of the prostate cancer group (22.56 ± 0.53) was significantly lower than the control group (24.49 ± 0.62) (P = 0.023).
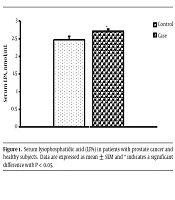
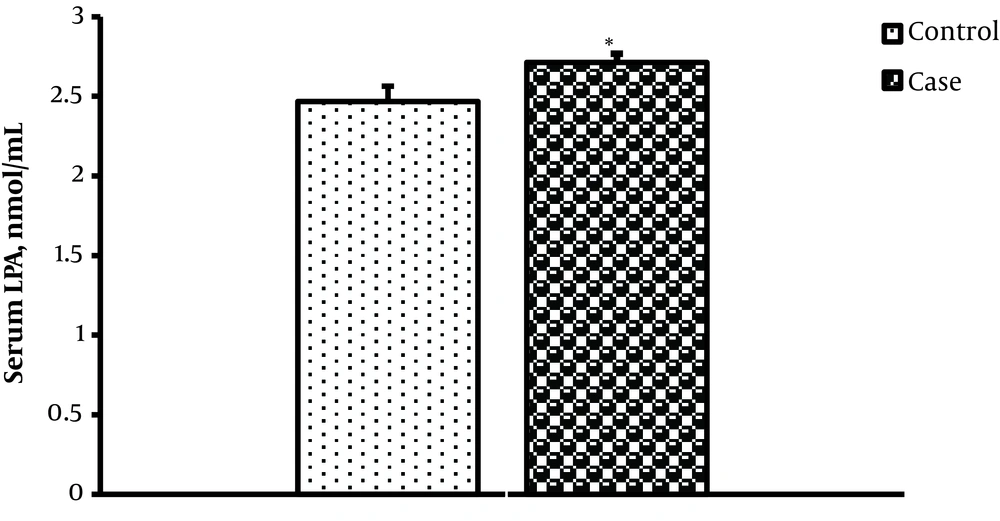
The mean LPA level (nmol/mL) of the prostate cancer group was significantly more than the control group (respectively 2.714 ± 0.054 and 2.467 ± 0.097) (P = 0.037) (Figure 1).
In the One-way ANOVA test, serum LPA levels (nmol/mL) of low and intermediate-risk patients (2.59 ± 0.05) were significantly lower than the high-risk patients (2.87 ± 0.07) (F = 10.28; P = 0.001).
Serum LPA had no significant correlation with PSA (r = 0.271; P = 0.248), Gleason score (r = 0.008; P = 0.974), and also with BMI (r = 0.251; P = 0.273). The amount of cut-off lysophosphatidic acid for prostate cancer was calculated as 2.58 nmol/mL; with this level, the area under the ROC curve was 0.70, the sensitivity was 80%, and the specificity was 61%.
5. Discussion
The findings of this study showed that the mean BMI in healthy subjects was higher than in patients with prostate cancer. Studies by Ruiz-Gracia et al. and Neary et al. report similar results (16, 17). In these studies, the BMI of patients with cancer has significantly decreased the pre-cancer status. This can be due to a loss of balance between the absorption and intake of calories in the body; either because of a lower caloric intake with normal consumption or normal absorption with an increase in the amount of calorie intake (18). This study aims to evaluate the serum lysophosphatidic acid level in patients with prostate cancer. The results of our study showed that serum LPA level was significantly different between the patients and the control group, as it was higher in the prostate cancer group than in the control group. The results of this study are confirmed by those of many studies of other cancers, including Panupinthu et al., Li et al., and Liu et al. studies (19-21).
In patients with ovarian cancer, it has been reported that there is an increase of LPA in the blood and peritoneum, which leads to the protection of ovarian cancer cells against chemical drugs that induce apoptosis. Studies on various tumor cells and cancer cell lines have shown that LPA is concerned with the etiology of cancer. Studies have also reported that in B cells LPA, acting as a growth factor, increase intracellular calcium, cell proliferation, and forming immunoglobulins (22, 23).
The LPA is supposed to mediate gene expression, especially in cancer cells, targeting VEGF, cyclooxygenase-2, growth-regulated oncogene alpha, Interleukin (IL)-6, IL-8, and urokinase plasminogen activator genes involving in angiogenesis, inflammation, and tumor progression (24-26). In tumors, VEGE causes vascular permeability as well as angiogenesis in cancerous tissues, which is known to be one of the most important angiogenic factors (27). The expression of mRNA and VEGF protein increases wildly in malignant tumors such as ovarian cancer (27). In ovarian cancer, VEGF has the main role in ascites by increasing vascular permeability (26).
The LPA promotes the expression of VEGF in cancerous ovarian cell lines as well as other cancers (28). Due to the abundance of LPA in ascites of ovarian cancer, according to the ability of LPA in the regulation of the VEGF expression, VEGF increases in cancer patients. This process includes the induction and activation of HIF-1α by the LPA (28). It was shown in another study that the lack of oxygen in the tissues associated with the effect of LPA on the regulation of the gene expression (29).
The LPA also involves in metastasis and invasion by increasing the expression of important mediators of these matters including urokinase-type plasminogen activator (uPA) and matrix metalloproteases (MMPs) (30). It is suggested that the cancer cell migration can be caused by LPA, via Rho/ROCK/actomyosin and Ras/MEKK1 pathways (31). These results have been detected in other tumors such as the pancreas, colon, stomach, liver, and prostate cancers (32, 33).
At least two main paths are considered for the production of LPA: hydrolysis of phosphatidic acid by phospholipase A1 and A2 and lysophospholipids hydrolysis, including hydrolysis of isophosphatidyl serine, and lysophosphatidyl choline by lysophospholipase ATX/D. It is believed that the first pathway operates with the presence of phosphatidic acids; hence it mainly occurs in the cell or on the cell membrane (34). Other LPA production pathways are glycerol 3-phosphate acylation by glycerophosphate acyl transferase and phosphorylation of monoacyl glycerol by monoacyl glycerol kinase. Nevertheless, these 2 routes seem to produce LPAs that are used as precursors for the synthesis of glycerol lipid instead of the source for molecules of extracellular signaling (35).
Considering the limitations of our study, this should be confirmed by conducting randomized studies and higher sample sizes.
5.1. Conclusions
The serum LPA levels increased in prostate cancer, suggesting that LPA may be considered as a biomarker in this cancer and take part in the regulation of the functions of prostate cancer cells as an autocrine/paracrine mediator.