1. Background
Pain is one of the most common and important symptoms in many acute and chronic diseases that brings the patient to physician attention (1, 2). Allopathic medicine usually has some side effects, high costs, and serious interactions with other drugs, and common anti-nociceptive drugs are no exception. Therefore, it is prudent to search for alternatives (3, 4). The annals of traditional Persian medicine (PM) are replete with valuable manuscripts and books containing priceless herbal experiences. Searching through them can lead to finding suitable herbs as painkillers. This approach has been emphasized by the World Health Organization (WHO) (5).
Ficus carica L. (Moraceae family), named as the common fig, is known as a medicinal herb with the analogous function as anti-nociceptive. In addition, different parts of this plant are used in traditional medicine to treat various ailments such as gastrointestinal (colic, indigestion, loss of appetite, and diarrhea), respiratory (sore throats, coughs, and bronchial problems), urinary, endocrine, reproductive and cardiovascular disorders and as an anti-inflammatory and antispasmodic remedy (6-11). In several studies, the anti-nociception effect of fig was investigated. In a previous study, it was determined that the hydroalcoholic extract of F. carica leaf contains analgesic effects in both pain phases (12). The acute and chronic anti-inflammatory and analgesic effects of the aqueous and alcoholic extract of fig leaf were ascertained in another study (13).
2. Objectives
Regarding the fact of investigated and approved studies about the anti-nociceptive effect of some fig’s species such as F. deltoidea and F. glomerata and and leaf extract of F. carica, we decided to study the anti-nociceptive effect of F. carica fruit (the most available parts of this plant) in this research (14, 15).
3. Methods
3.1. Plant Materials
Fruits of Ficus carica, being collected from a reputable store, were saved in the herbarium of the School of Pharmacy, Shahid Beheshti University of Medical Sciences.
3.2. Preparation of Aqueous Boiled Ficus Extract
Ficus carica fruits were cleaned, dried, and powdered. Distilled water was added to this powder with a ratio of seven to one. After 15 minutes of boiling, the achieved solution was filtered (Whatman 11 cm Qualitative Lat No. 100111) and concentrated (Ben Marry).
3.3. Preparation of Ether De-Petroleum Extract
After cleaning, drying, and powdering of F. carica fruits, achieved powder was mixed with ethanol 80% or ether de-petrol (Merck). The obtained solution was concentrated (Rota Evaporator) after filtration (Whatman 11 cm Qualitative Lat No. 100111).
Water and alcoholic extracts of F. carica fruits were used to make different concentrations of solutions by use of distilled water.
3.4. Animals
Wistar male rats (weight of 230 to 330 g) were used in this survey (Physiology Department of TUMS). They were housed in plexiglass cages (each group consists of six rats) with free access to the food and water on a 12 hours light and 12 hours dark cycle, ambient temperature (22 to 25 degrees Celsius).
3.5. Formalin Test
Dubuisson and Dennis’s method was used for formalin test (16). The pain was established by subcutaneous injection of formalin in rat paw as testing grounds. The dosage of 0.05 cc of formalin 2.5% was used. The negative control group received distilled water (DW) and the positive control group was treated with the overall volume of 2.5 mL intraperitoneal (i.p.) sodium salicylate (SS, Merck). These dosages were determined based on mg/kg. Experimental groups were treated with different doses of aqueous boiled and petroleum ether extracts of F. Carica. The first part of the study was carried out on four groups of six rats with aqueous boiled Ficus extract in various doses (500, 1000, 2000 mg/kg) and being compared with distilled water (DW) in intraperitoneal injections. The second part was done on three groups of six rats with petroleum ether extract in various doses (500, 1000 mg/kg) and being compared with DMSO the same as part one. Drugs were injected 30 minutes before formalin injection. Then, the severity of pain was assessed by formalin test every 15 seconds for one hour. Average pain in the first 10 min after formalin injection was considered as the acute phase and the time between 16 to 60 minutes were evaluated as the chronic phase. The pain rating scale was simplified by means of using only licking behavior of the injected paw as an indicator of pain response (17).
3.6. Statistical Analysis
The results of the experiments are expressed as mean ± SEM. The differences were computed by means of one-way ANOVA and the Tukey test, which were used in case of a statistically significant. For the P < 0.05, the difference was considered significant.
4. Results
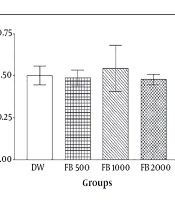
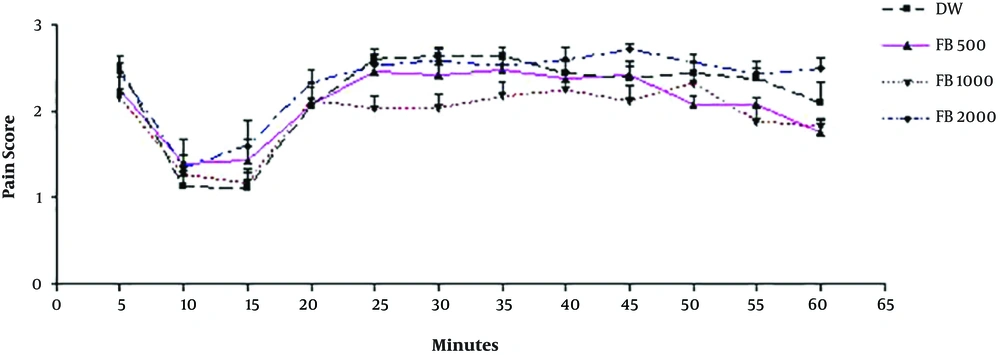
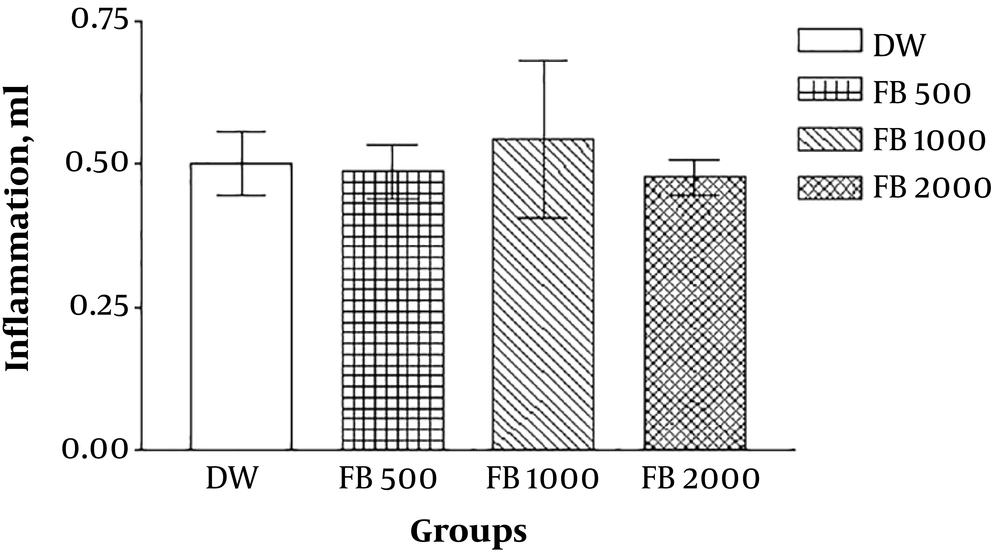
In the first part of the study, no significant difference was recognized between the aqueous boiled Ficus extract in various doses (500, 1000, 2000 mg/kg) and DW (P > 0.05). In other words, the aqueous boiled extracts with applied doses are without analgesic effect (Figure 1).
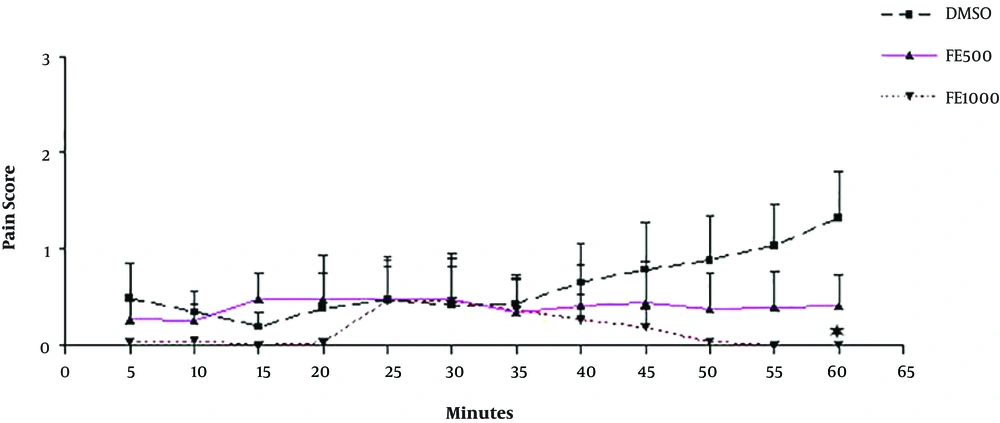
The result of the second part of the study determined a significant difference between 1000 mg/kg of the petroleum ether extract and the DMSO group in the last five minutes of the study (P < 0.05). However, the petroleum ether extract of F. carica with the doses used does not have an anti-nociceptive effect (Figure 2).
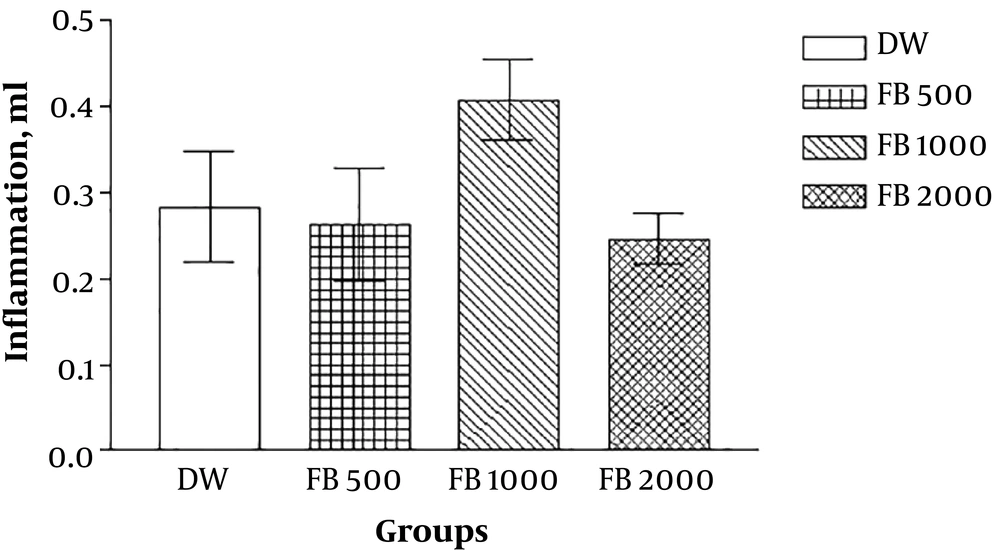
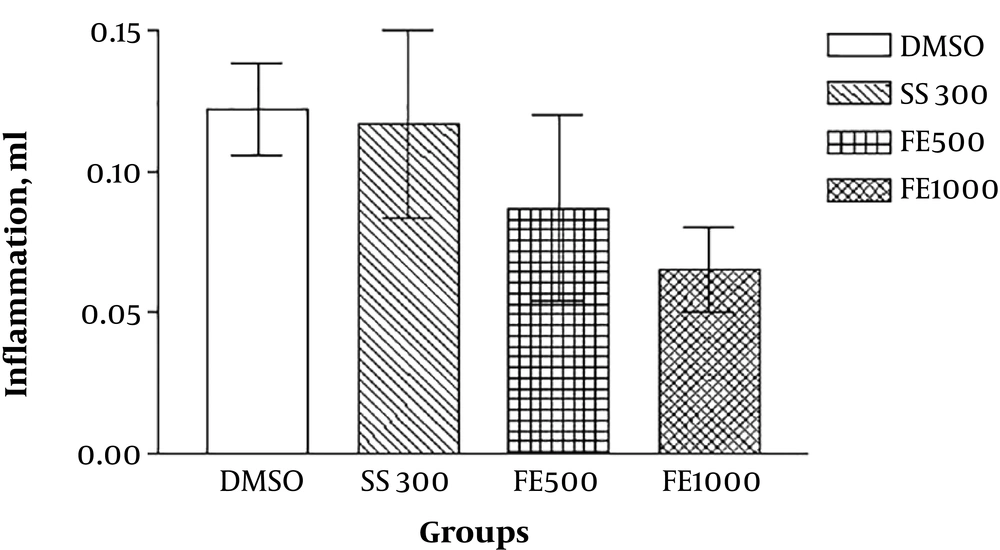
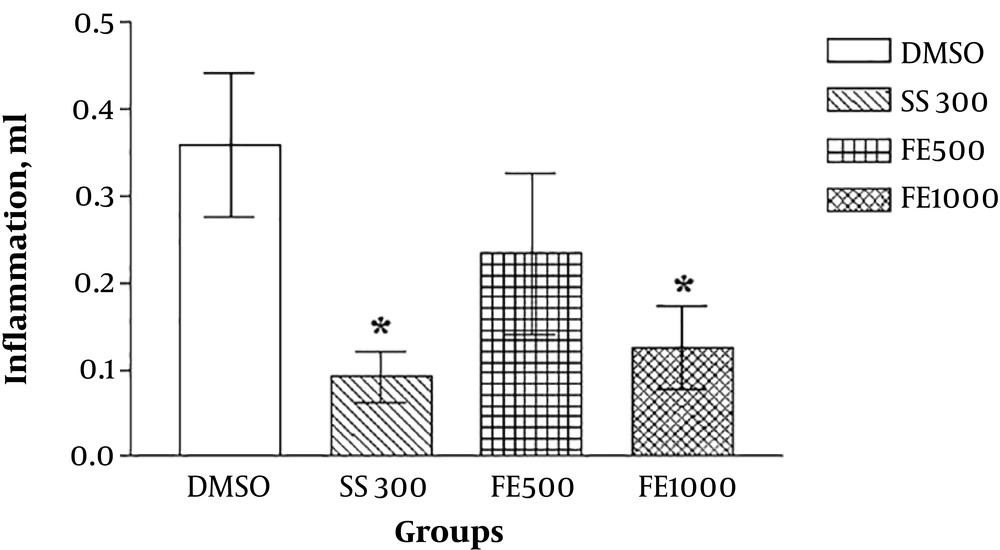
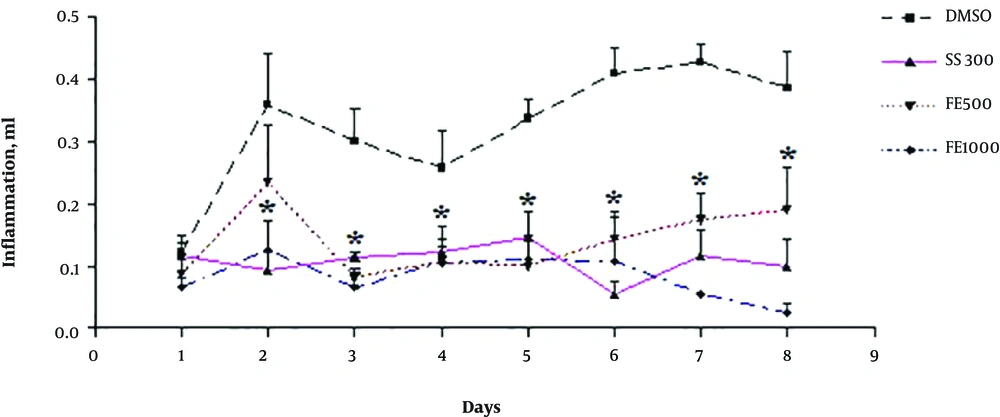
The results of the lateral experiments that evaluated the anti-inflammatory effects of the extracts by formalin-induced pellet edema showed that the aqueous and ethanolic extract of Ficus fruit has no anti-inflammatory effect and the petroleum ether extract has a dose-dependent (1000 mg/kg) anti-inflammatory effect even in comparison with sodium salicylate (Figures 3 - 7).
The rate of inflammation at the second day of experiment with ether de-petrol F. carica extract (mean ± SEM). * Significantly difference with control group: P < 0.05. DMSO, dimethyl sulfoxide; SS, sodium sulfate (300 mg/kg); FE, Ficus petroleum ether extract with different doses (500, 1000 mg/kg).
The rate of inflammation at the whole test period of experiment (8 days) with ether de-petrol F. carica extract (mean ± SEM). * Significantly difference with control group: P < 0.05. DMSO, dimethyl sulfoxide; SS, sodium sulfate (300 mg/kg), FE, Ficus petroleum ether extract with different doses (500, 1000 mg/kg).
5. Discussion
The anti-nociception effects of some plants have been investigated comprehensively. The analgesic effect of Ficus carica L. has been emphasized in Persian medicine (8). According to the results of previous studies, F. carica showed a variety of properties including anti-oxidant (18), anti-mutation (19, 20), cytotoxic and anti-cancer (21), tumor necrosis and inhibitor (22, 23), anti-diabetes (24, 25), hypolipidemic activity (24-27), blood coagulant (28), anti-worm (29), anti HSV-1 (30), topical treatment of wart (31), and the irritant potential of triterpenoids from F. carica leaves (32).
Except for a limited report of the anaphylactic reaction (33, 34) and photodermatitis (35) associated with its leaf syrup, no other complication was reported for this plant.
According to the detailed search results for the analgesic effect of F. carica, several studies had been performed on the different extract of its leaf. Anti-inflammatory and anti-nociception effect of the aqueous and alcoholic extract of leaf figs were investigated by Masalegou and et al. Achieved results indicated the dose-dependent acute and chronic analgesic and anti-inflammatory effects (13). Based on Arzi et al., hydroalcoholic extract of fig leaves were effective in both the acute and chronic phases of the pain (12).
In our study, the extract of fig fruit with the formalin test was carried out in the rat.
The result of LD50 showed that the aqueous boiled and alcoholic extract of F. carica fruits were non-toxic and non-lethal, however, the petroleum ether extract is toxic and deadly (LD50 level of this extract is about 1000 mg/kg). Therefore, doses of less than 500 mg/kg should be used to prevent mortality, as this dose contributed to about 15% mortality.
Due to the lack of analgesic effect in the 2000 mg/kg doses of aqueous extract and the inappropriate use of higher doses in research and clinical treatment, it can be concluded that the aqueous extract has no analgesic effect. In other words, the water solvent does not remove analgesics from fig fruits.
The petroleum ether extract of F. carica fruits (500 and 1000 mg/kg) also did not show a clear anti-nociceptive effect during the test. Just at the end of the experiment, in the last five minutes, the difference between the 1000 and control groups shows a significant dose-dependent delayed analgesic effect. These findings indicated that in substances isolated from the plant by ether petroleum solvent has little or no analgesic effect or their work begins with a delay.
In the present study, the anti-inflammatory effect of the extracts was also investigated by formalin-induced pellet edema using a plethysmometer. The results indicated that the aqueous and ethanolic extracts of Ficus fruits have no anti-inflammatory effect and the petroleum ether extract has a dose-dependent anti-inflammatory effect even in comparison with sodium salicylate. Therefore, it can be concluded that some of the active substances present in petroleum ether extract have anti-inflammatory effects and, to a lesser degree, has analgesic effects. Ether de-petroleum solvent can separate nonpolar compounds such as steroids, terpenoids and water-insoluble amines from plants and probably one of these compounds in fig fruit, isolated by solvent, causes the property mild anti-nociception and strong anti-inflammatory effect of petroleum ether Ficus extract. In other words, the petroleum ether extract is likely to have a steroid that has been able to control inflammation well but has no clear or rapid effect on pain control and this can be investigated by analyzing the extract and reaching its components and repeating experiments with extract fractions.
5.1. Conclusions
It was concluded that Ficus carica fruits aqueous extract (500, 1000, 2000 mg/kg) doesn’t have any anti-nociceptive and anti-inflammatory effects on formalin induced pain in the male rat paw. But petroleum ether extract has a delayed anti-nociceptive effect dose-dependently and a dose-dependent anti-inflammatory effect.