1. Background
The animal gustatory system is responsible for life, especially nutrition and propagation. Taste sensation and response to the tastants are essential to start neuroendocrine web working and regulate metabolic procedures (1). Taste is one of the important chemical senses to identify nutritive materials choosing or avoiding (2). Taste buds play a major role in the recognition of energy sources (3). Taste signals are transmitted to the central nervous system through the chorda tympani nerves, which carry sweat taste information from taste buds of fungiform papillae to taste sense-integrating higher brain centers. It is well known that the geniculate ganglion neurons have bipolar extensions, one of which is linked to taste buds and the others are linked to brainstem/cortex hardware (4). The chorda tympani nerve is informed with sugar stimulation of taste buds in the anterior tongue and sends that information to geniculate ganglia, nucleus tractus solitarius, parabrachial nucleus, deep brain, amygdala, hippocampus, thalamus, internal capsula, insula, and somatosensory cortex (5). Gustatory network injuries can cause taste buds atrophy and impaired taste sensation (6). Chorda tympani nerve transection results in morphological changes to fungiform papillae and associated taste buds (7). The insular cortex modulates taste functions (8) because the primary gustatory area is located in the insular cortex (9).
Although diabetes mellitus may occur as a result of taste disturbance affected by neurocellular degeneration of taste regulating network, Cheng et al. (10) showed that the taste bud cell apoptosis might contribute to type 2 diabetes-like disease. It is unknown that diabetes mellitus is a causative agent or the result of taste disturbance. If the tongue can play a role in peripheral glycemic imbalance (11), we easily theorize that taste bud connecting network insufficiency or injury may rely on hyperglycemia, which has not been mentioned in the literature so far. This is because hyperglycemia is frequently seen following a stroke in diabetic/non-diabetic patients (12) and acute post-stroke hyperglycemia results from insular cortex ischemia (13). Aydin et al. (14) showed that subarachnoid hemorrhage (SAH)-induced insulo-vagal complex ischemia should be considered a causative agent during SAH. The taste sense is obtained from taste buds in the oral cavity, intestine, pancreatic β-cells, and glucose-responsive neurons in the brain (3), all of which start insulin secretion required signals and send them to the pancreas. Cheng et al. (10) showed that cell apoptosis of taste buds in circumvallate papillae may contribute to taste disturbance in diabetes.
2. Objectives
The present study showed that the taste bud-facial network could be responsible for blood glucose regulation. If it is not so, facial ischemia-induced taste bud degeneration could not result in hyperglycemia following SAH.
3. Methods
3.1. Experimental Design and Animal Selection
This experiment was conducted on 32 rabbits (2.5-years-old, 4.5 ± 0.5 kg) obtained from the Experimental Animal Laboratory of Ataturk University. The blood glucose levels were measured at the beginning of the experiment. The animals were housed in standard laboratory conditions. The blood glucose levels were measured at the beginning, mid-phase, and the end of the experiment. The animals were divided into the groups of control (n = 5), physiologic serum saline (SHAM; n = 5), and subarachnoid hemorrhage with 0.5 cc homolog blood injection into cisterna magna (study; n = 22) three times a week that were sacrificed under general anesthesia after two weeks. The blood glucose level of 113 ± 20 mg/dL was accepted as normal (G-I; n = 11), lower than 80 mg/dL as hypoglycemic (G-II; n = 6), and higher than 149 mg/dL as hyperglycemic (G-III; n = 5). After two weeks, all animals were decapitated under general anesthesia following intracardiac formalin injection. Their tongues and facial nerve complexes, together with geniculate ganglia, were extracted bilaterally. Their normal and degenerated neurons of geniculate ganglia were examined by stereological methods.
3.2. Statistical Analysis
The statistical analysis was done only between glucose levels and degenerated neuron density of geniculate ganglia using Kruskal-Wallis and Mann-Whitney U tests although we examined taste buds of the tongue that will be published as another article in the same journal. We accepted P > 0.005 as non-significance.
3.3. Tissue Preparation
The tongues, facial nerves, and geniculate ganglia were gently removed and rapidly fixed in a 10% formalin solution for two days and embedded in paraffin blocks. The sections were stained with H & E and GFAP immunostaining techniques. The samples were sectioned via Leica RM2125RT microtome (Leica Microsystems, Wetzlar, Germany). To determine and compare the histological architectures of the geniculate ganglia, all geniculate ganglia were examined by stereological methods. Degenerated neuron density of geniculate ganglia and glucose levels were analyzed statistically.
4. Results
4.1. Clinical-Anatomical Findings
Two animals died in the experimental period because of cardiorespiratory arrest and hyperglycemia. Electrocardiographic disturbances were detected as bradycardia, bigeminy-trigeminy beats, inversed P-T waves, and QRS abnormalities. Glasgow comma scales changed between 7 and 12 right after the experiment and were renormalized on the second day of the experiment in the study group. Macroscopical examinations of brains showed bloody-clotted subarachnoid spaces, Petro-clival cisterns just around the facio-cochlear nerve entering zones to the internal meatus, pia-arachnoid adhesions, arachnoid inflammation, edematous cerebellum, and brainstem.
4.2. Histopathological Findings
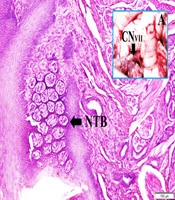
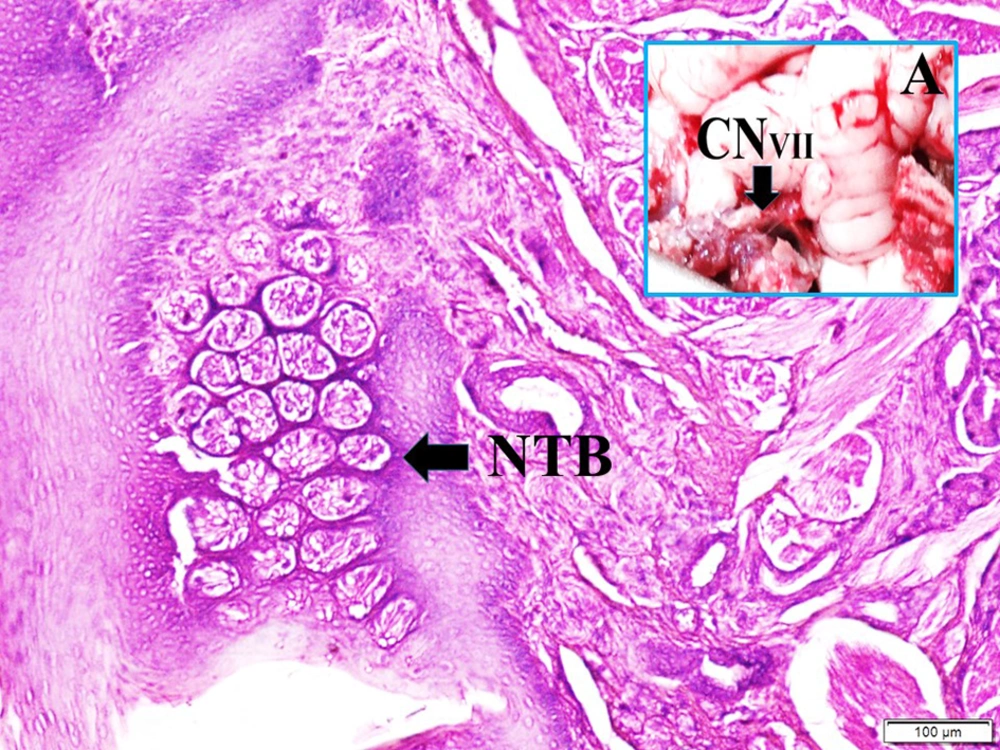
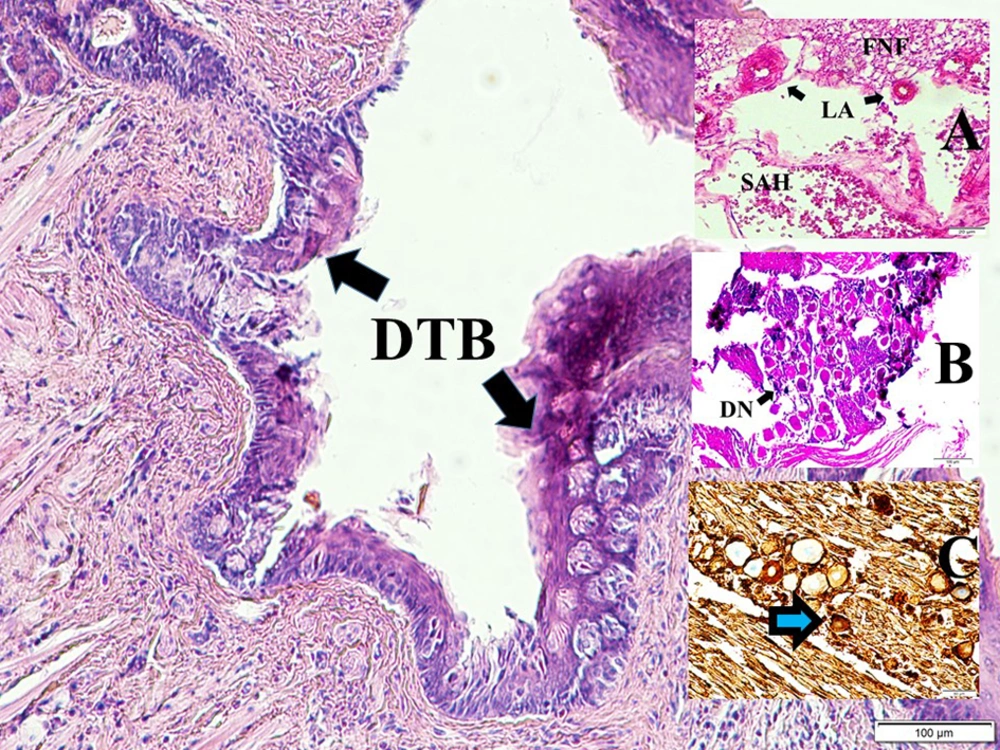
Figure 1 shows the anatomical view of the facial nerve and internal auditory artery just entering internal meatus and normal taste buds in a normal rabbit. The histopathological appearance of the bloody pontocerebellar cistern, constructed labyrinthine artery branches among facial nerve fibers, degenerated geniculate ganglion neurons, and deformed shrinkage neurons of the geniculate ganglion, and partially deformed taste buds-induced facial nerve ischemia are seen in a SAH created rabbit (Figure 2).
Histopathological appearance of bloody pontocerebellar cistern (SAH), constructed labyrinthine artery (LA) branches among facial nerve fibers (FNF) (LM, H & E, 40×/A), degenerated geniculate ganglion neurons (DN) (LM, H & E, 10×/B), and deformed shrinkage neurons of geniculate ganglion (blue arrow) (LM, GFAP, 20×/C) and partially deformed taste buds (DTB) induced facial nerve ischemia are seen in a SAH created rabbit (LM, H & E, 10×/Base).
4.3. Numerical Results of Experiment
The mean normal blood glucose level was 115 ± 9 mg/dL before surgery. The pre-sacrificed glucose levels were 113 ± 8 mg/dL and the neuron density of the geniculate ganglia was 7.421 ± 530 /mm3. The degenerated neuron density of geniculate ganglia was 13 ± 4/mm3 in control, 21 ± 7/mm3 in SHAM, 27 ± 7/mm3 in G-I, 21 ± 5/mm3 in G-II, and 112 ± 18/mm3 in G-III groups. The P values of glucose levels-degenerated neuron density of geniculate ganglia between control/G-III was: P < 0.00001; SHAM/G-III: P < 0.0005; “GI/GII: P < 0.005.
5. Discussion
5.1. General Considerations
The mammalian taste system is required for feeding and propagation (1). The sweet taste signals of taste buds are carried to the central nervous system via chorda tympani, a branch of facial nerve innervating the taste buds of the anterior tongue (6). A significant reduction in the sweet taste sensation occurs because of dangerous degeneration in taste buds, geniculate ganglia, and nucleus tractus solitarius following chorda tympani injury (5). The geniculate ganglion cells have bipolar extensions linked to taste buds and brainstem connections. Central taste network localized within the nucleus of the solitary tract, the parabrachial nucleus of the pons, ventral, and medullary reticular formation, a caudal brainstem pathway is leading to reflexive motor functions (4). Geniculate ganglia network is linked with dorsal vagal complex, parabrachial nuclei, and lateral part of the insular visceral sensory cortex (15). Parabrachial nuclei make extensive synaptic connections with zona incerta, internal capsule, ventral forebrain, amygdala, bed nucleus, hypothalamus, preoptic nucleus, innominate substance, of Broca’s diagonal band, septum mediale, tuberculum olfactory, gustatory cortex make (16, 17), thalamus, claustrum, dorsal agranular insular, and the dysgranular insular cortex. Historically, the insula was considered the primary gustatory cortex (18). Bilateral gustatory cortex lesions significantly impair taste sensitivity (19). The aging process affects the human taste system at peripheral and central levels (20). Damage to the gustatory cortex in rats has been reported to impair taste recognition and eating behavior severely (21).
5.2. Diabetes and Gustation
Gustation is one of the important chemical senses to identify toxic chemicals (2). The sweet taste receptor plays an important role in recognition and carbohydrate metabolism (3). The taste bud degeneration may contribute to taste disturbance and type 2 diabetes development (10). The lacrimal gland dysfunction may be correlated with motor part palsy of the facial nerve, but taste sensation disturbance is not correlated with facial nerve motor palsy (21). Although subarachnoid hemorrhage induces dry eye, there is no information if facial nerve palsy causes hyperglycemia following SAH (22). Probably, this study is may be the first stud on facial ischemia-induced hyperglycemia following SAH.
5.3. Brain Diseases and Diabetes
Hyperglycemia is common after stroke in diabetic and non-diabetic patients. Lower brainstem nuclei of the facial-vagal nerve modulate insulin secretion and are usually affected in cerebral infarcts (12). Insular lesions may aggravate hyperglycemia following SAH (23). It is becoming apparent that there is a strong link between taste perception and glucose homeostasis (11). Diabetes can decrease the ability of individuals to detect and recognize sweet, salty, and bitter tastes (24). The decrease in fungiform papillae related to decreased taste sensation could result in neurodegenerative disease (25). However, we showed that taste information-carrying neural mechanism disorders may cause diabetes-like disorders.
5.4. Limitation
This study should include more biochemical analyses, radiological examinations, and electrophysiological studies.
5.5. Conclusions
In summary, we hypothesized that taste buds of the tongue might determine the glucose concentration in the foods and stimulate taste fibers of geniculate ganglions. Stimulated geniculate ganglion neurons could inform insulin-secreting branches of vagal nerves to secrete insulin from Langerhans islands. Therefore, the higher degenerated neurons of geniculate ganglia cannot determine glucose concentration in the foods and insufficient vagal stimulation can cause inadequate insulin secretion from the pancreas. Hyperglycemia may be inevitable during SAH, which has not been mentioned in the literature.