1. Background
Stressful experiences are usually manifested through their effects on the activation of several mechanisms, which are linked to the onset of a variety of affective disorders. In this regard, most of the previous studies have administered Pavlovian fear conditioning with the aim of understanding and controlling anxiety in humans (1, 2). Since excessive fear and anxiety have led to the loss of performance, and even anxiety disorders in many people around the world, many researchers have focused on the neurobiological mechanisms involved in the extinction of conditioned fear. Fear extinction is a process in which frequent conditioned stimuli lessen the expression of conditioned fear responses in the absence of the unconditioned aversive stimulus (3). At the behavioral level, it is shown that extinction does not mean to erase the original fear memory, but it means creating a new inhibitory or suppressive memory to compete with the expression of original fear memory (4). Fear extinction has been well defined at the behavioral level. However, there is still relatively little knowledge about its neural substrates, including the regions and molecular processes whereby extinction-related plasticity arises. Nevertheless, in the last decade, sufficient evidence suggested that several brain structures form the fear and extinction network, including the amygdala, prefrontal cortex, and hippocampus (5, 6).
Several studies assessed the effects of deep brain stimulation (DBS) to the basolateral amygdala (BLA) on defensive burying with the hypothesis that the reduction of this behavior is equivalent to anxiolytic (7-10). For instance, Langevin et al. (2010) assessed the defensive burying by a ball (a conspicuous object) in rats following the BLA DBS in a sham-controlled study. They reported a statistically significant less time for burying the ball in the DBS-treated rats than sham controls. However, we still know little about the mechanisms involved in the anxiolytic effects of BLA DBS. Although the amygdala has been considered a critical structure for fear processing, conclusive evidence defines an important role for the medial prefrontal cortex (mPFC) as well. Some researchers suggested the contribution of protein kinases, gene expression, and protein synthesis in the amygdala as well as in the mPFC in the extinction process (11-13). Evidence demonstrates upregulation of early gene c-Fos in the mPFC immediately following the extinction training, which is not a consequence of the acquisition of fear conditioning (13, 14). Thus, mPFC has also been targeted to investigate the mechanisms responsible for fear processing. It seems that the plasticity within the infralimbic (IL), particularly that engages brain-derived neurotrophic factor (BDNF), is associated with extinction learning. Therefore, most trials in this region have focused on the IL subregion (15, 16). It has been shown that IL DBS inhibits the amygdala central nucleus and; therefore, reduces the expression of conditioned fear (5). On the other hand, from previous studies, we know that the prelimbic (PL) subregion of mPFC is critical for the expression of learned fear through projections to the BLA (17). Although many efforts have been made to target the IL, little is known about the extinction-related plasticity within the PL.
2. Objectives
Recent evidence suggests that high frequency of DBS may inhibit hyperactivity in the BLA, followed by suppression of the fear experience and enhancement of the extinction process (1, 9). Therefore, the current study investigated whether DBS in the PL or BLA might separately modify the extinction process and affect the fear-related behaviors in a rodent model. For this purpose, we compared the effects of DBS of BLA versus PL in terms of freezing behavior, c-Fos protein expression, and serum corticosterone levels.
3. Methods
3.1. Subjects
Thirty-five male Wistar rats were used in this study, weighing 220 - 250 g. Each rat was housed in a transparent polyethylene cage located in the animal houseroom. At first, the animals were kept in cages on 12 hours of light and 12 hours of darkness with free access to food and water. Then, they were randomly assigned to five groups (n = 7), named after their procedures, as follows: negative control group (NC; no manipulation), sham surgery (Sham; surgery circumstance without electrode implantation), positive control group (PC; surgery + fear conditioning + no treatment), BLA-DBS group (BLA; surgery + fear conditioning + DBS treatment in BLA area), PL-DBS group (PL; surgery + fear conditioning + DBS treatment in PL area). This study was approved by the Ethics Committee of Tehran University of Medical Sciences with code IR.TUMS.REC.1394.1862.
3.2. Surgery
To anesthetize the animals, 30 mg/kg of ketamine and 15 mg/kg xylazine were used as an intraperitoneal injection and, then, they were placed into a stereotaxic apparatus (1). After placing both levels of Lambda and Bregma in a horizontal line, one hole over the right PL or BLA and three additional holes, for anchoring screws, were drilled with a dental drill. An exciting electrode was placed unilaterally in the BLA or PL. According to the Paxinos and Watson Atlas, we specified and marked the area of infusion (1987). The coordinates of the location of the guide cannula in the PL are as follows: 2.9 mm anterior, 1.0 mm lateral, 4.2 mm ventral relative to bregma, and in the BLA: -2.5 mm anterior, 4.8 mm lateral, and 7.4 mm ventral relative to bregma.
Dental cement was used to fix the connector and four screws onto the surface of the skin, so the arms of the stereotaxic device were released. Seven days to rest were considered for the animals after the operation.
3.3. Fear Conditioning
As stated in the previous study, we used a rodent observation context (30 × 37 × 25 cm), which was situated inside a soundproof chamber for training the rats and behavioral testing. Each observation context as the conditioned stimulus (CS) was cleaned before and after each test with a 90% ethanol solution. On the conditioning day, the animals received shock according to the protocol described in the previous study (1). To evaluate the extinction and fear conditioning test (retention), after 10 days of DBS or resting, the rats were returned to the context with no electrical foot shock, remaining there for eight min. Their behavior was recorded during both training and testing (1). Then, the scores of each group were evaluated separately (18).
The first day after contextual fear conditioning (CFC), we started high-frequency DBS treatment according to the technique described in the previous study (1). The animals in the PC group were in the same condition while delivering no pulse.
3.4. Corticosterone Measurement
Ten days after DBS treatment and checking freezing behavior, 2 ml of blood was taken from the orbital sinus of the rats' eye (19) to analyze corticosterone levels (in each group). To separate the serum, blood samples were centrifuged at room temperature at 3,500 rpm for five minutes. Then, the serum was kept at -80°C. Serum corticosterone was measured by the ELISA technique (Cat. No. EIA4164).
3.5. c-Fos Expression Measurement
At the end of the experiment, after sacrificing the rats and removing the whole brain tissue promptly, and both BLA and PL were neatly dissected. The expression level of c-Fos protein in the BLA and PL was determined by Western blotting. The protocol was done according to our previous study (1).
Comparing the c-Fos band with the beta-actin band helped for quantification, and densitometry values were calculated for all experimental groups using Image-J software (1).
3.6. Data Analysis
According to the raw data obtained from these observations, the quantitative findings were expressed as mean ± standard error of mean (S.E.M). For quantitative data, analysis of variance and supplementary Tukey test were used to compare group differences. P < 0.05 was considered statistically significant.
4. Results
4.1. Tissue Confirmation
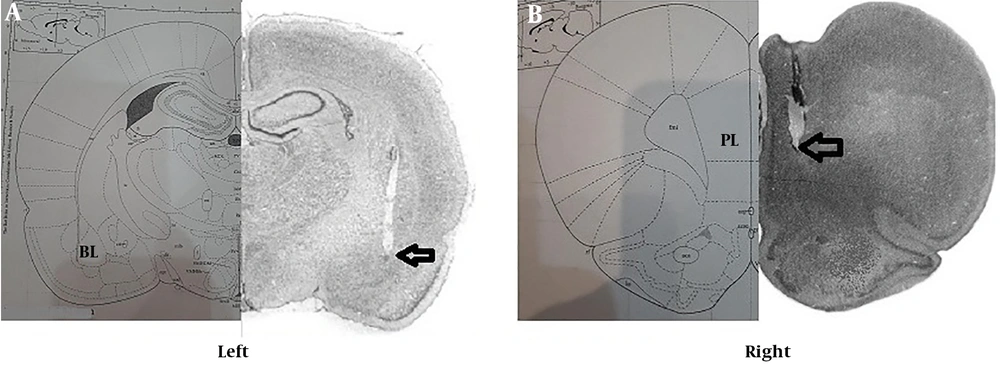
To evaluate the exact point of the electrode in the PL and BLA, after sacrificing the animals, brain slides were used to confirm the correct location of the cannula in these areas (Figure 1A and B).
4.2. Freezing Behavior
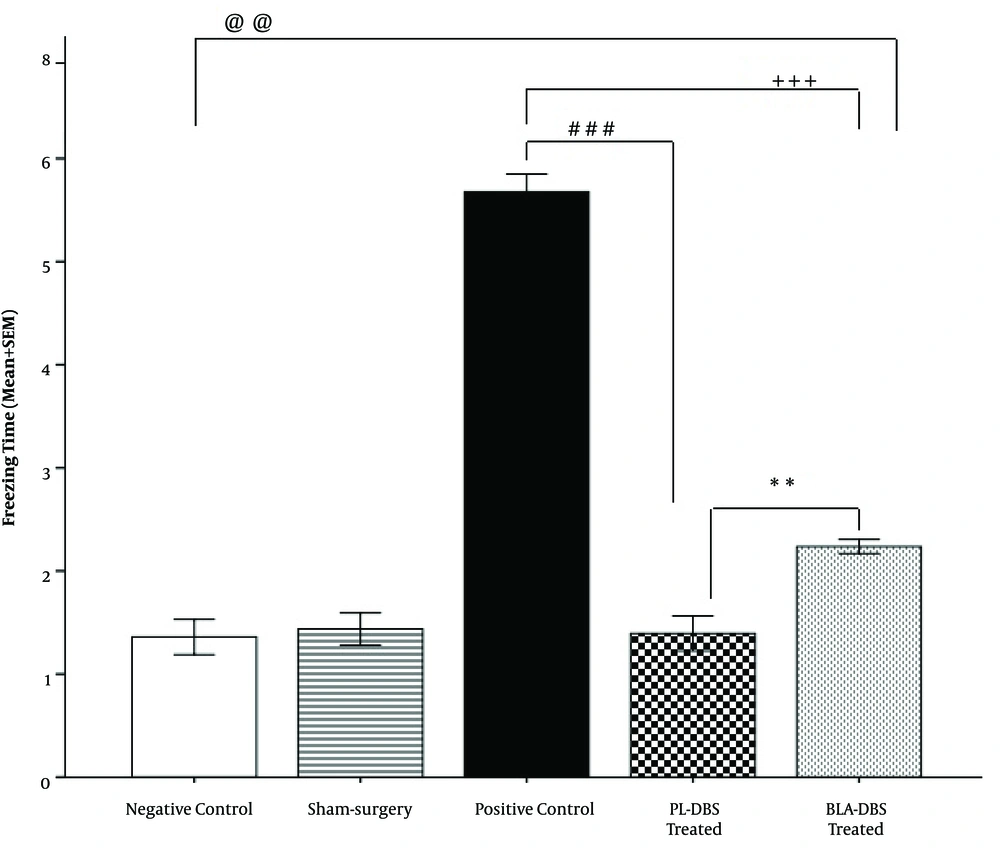
Freezing behavior was observed when fear-conditioned animals were re-exposed to the context after 10 days of resting time or DBS treatment. According to the results, the recorded freezing mean time in the PL group was significantly lower compared to the BLA group (P < 0.01) and the PC group (P < 0.001). The results were reported as mean time (minutes) spent in freezing posture ± SEM. There were significant differences among the PL group (1.3 ± 0.44), the BLA group (2.2 ± 0.06, P <0.01), and the PC group (5.6 ± 0.17, P < 0.001). There was also a significant difference between the BLA group (2.2 ± 0.06) and the PC group (5.6 ± 0.17, P < 0.001). Additionally, the results revealed that there was no significant difference between the NC group (1.30 ± 0.17) and the PL group (1.3 ± 0.44), unlike the BLA group (2.2 ± 0.06, P < 0.01) and the PC group (5.6 ± 0.17, P < 0.001). Because no significant difference was observed between the Sham group (1.40 ± 0.15) and the NC group (1.30 ± 0.17), its outcomes are not reported here (Figure 2).
4.3. Serum Corticosterone Levels
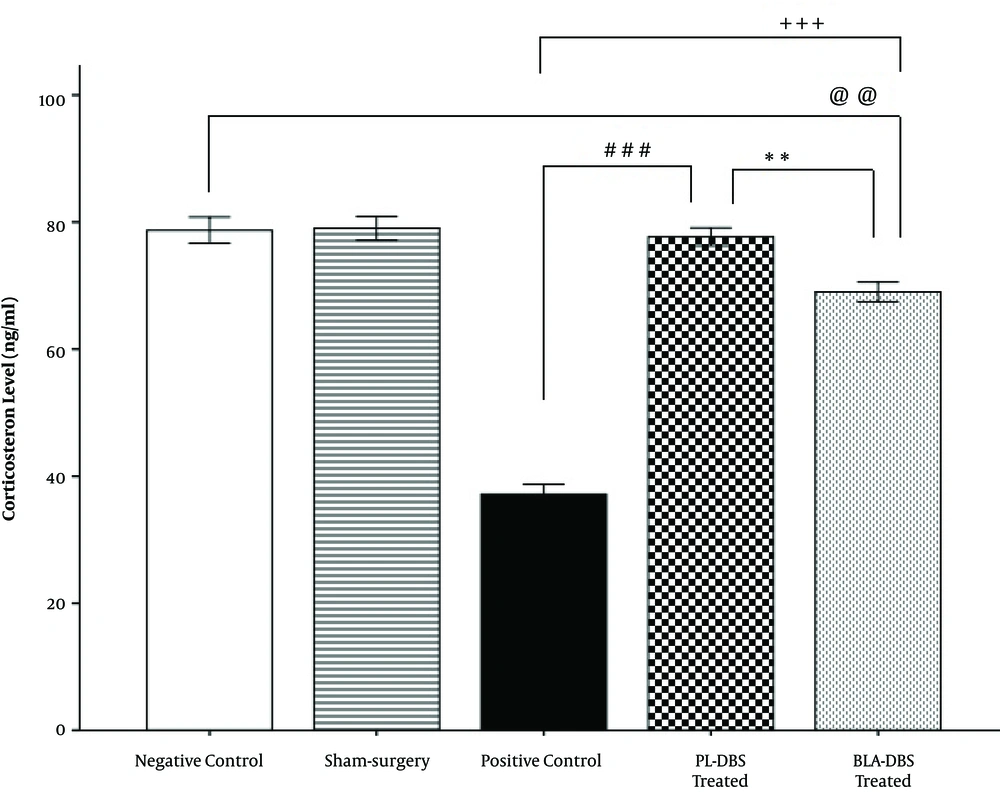
Blood serum corticosterone results indicated that the PL group (78.28 ± 1.47) had significantly higher serum corticosterone levels compared with both of the BLA (69.55 ± 1.6, P < 0.01) and PC groups (36.75 ± 1.6, P < 0.001). Additionally, the results revealed that there was no significant difference between the NC (77.21 ± 1.61) and PL groups (78.28 ± 1.47), unlike the BLA group (69.55 ± 1.6, P < 0.01). However, the BLA group (69.55 ± 1.6) had significantly higher corticosterone levels compared with the PC group (36.75 ± 1.6, P < 0.001). No significant difference was observed between the Sham (79.03 ± 1.8) and NC groups (77.21 ± 1.61) (Figure 3).
4.4. The c-Fos Protein Expression
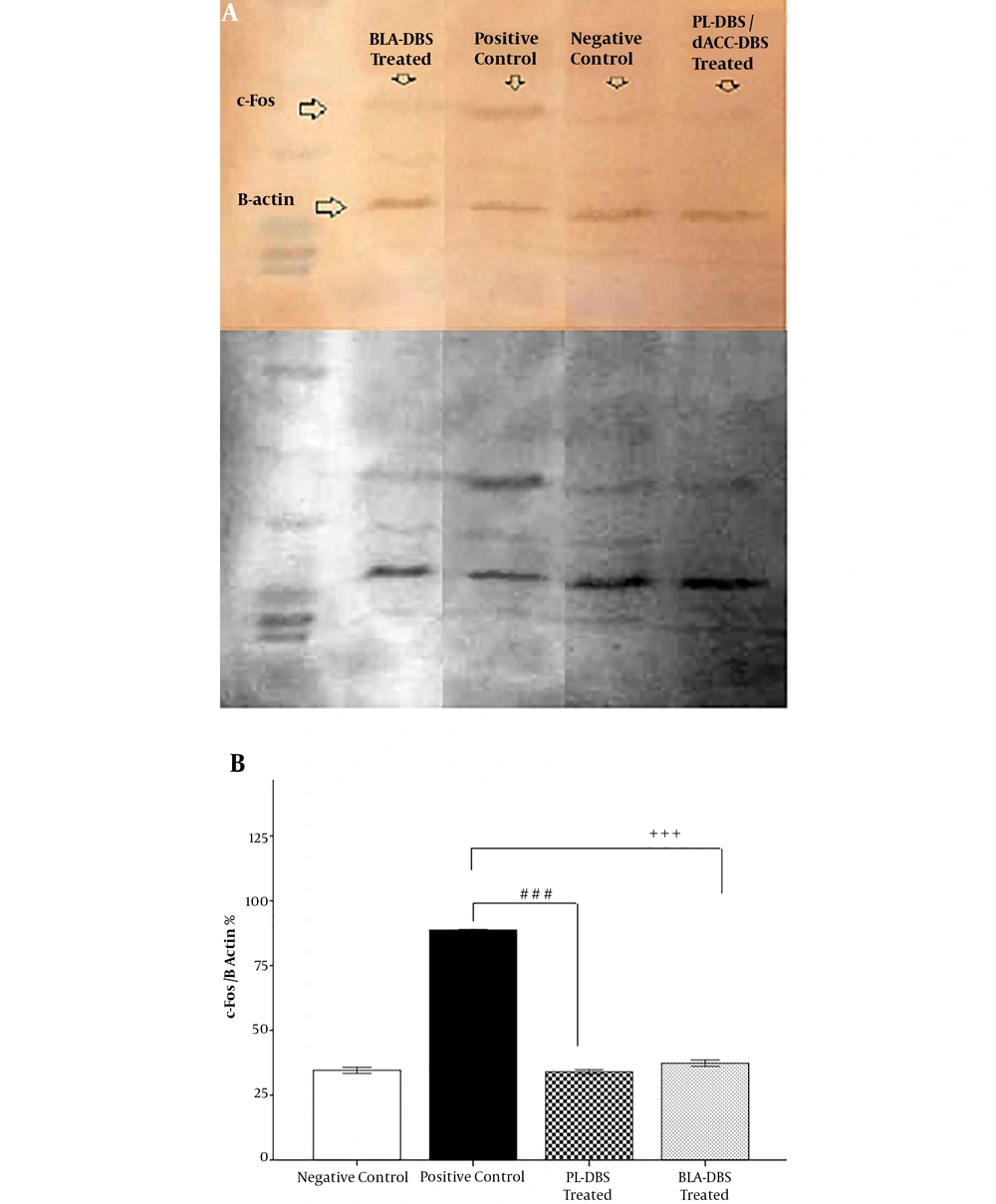
Western blotting was used at the end of the experiment to evaluate c-Fos expression. Statistical analysis indicated that there was no significant difference between the PL (32.98 ± 0.87) and BLA groups (36.32 ± 1.18) compared with the NC group (31.55 ± 1.12). On the other hand, the PL group (33.98 ± 0.87) showed a significant difference with the PC group (86.76 ± 0.02, P < 0.001). In addition, the BLA group (36.32 ± 1.18) revealed a significant reduction in c-Fos expression compared with the PC group (86.76 ± 0.02, P < 0.001) (Figure 4A and B).
5. Discussion
In this study, we examined the effect of high-frequency deep-brain stimulation on fear extinction in a CFC model focusing on the PL and BLA areas. The biological disturbances derived from conditional fear modeling are due to hyperactivity in the PL and the amygdala (20). These disturbances include avoidance behavior (freezing) (9), corticosterone/cortisol reduction (21), and increased expression of c-Fos (1, 22, 23). Our main findings support the facilitator effect of high-frequency DBS, particularly in the PL compared to the BLA, on conditioned fear extinction by reducing the freezing time and induction in the corticosterone hormone. Previous studies have also confirmed the ability of high-frequency stimulation to inhibit the hyperactivity of the PL and BLA, and they have proposed this technique for changing the PL and BLA activity trends (1, 7, 9). However, fear extinction was facilitated more by DBS in the PL than BLA, in this study. Given the reciprocal connections between the PL and BLA in the fear and extinction network (5), we can assume a reduction in BLA hyperactivity through the PL stimulation, leading to a double effect on enhancing the fear of extinction. In fact, the outcomes of the current study confirmed this assumption and indicated the dual effect of PL stimulation in extinction enhancement.
Freezing behavior is caused by fear in animals, while the amygdala moderates fear. Indeed, freezing behavior is a consequence of the strong amygdala inhibitory effect on the striatum (24). Additionally, studies have shown that there is a direct correlation between the hyperactivity of the PL and the intensity and duration of freezing behavior (20, 25, 26). Moreover, previous findings have indicated that high-frequency DBS has the ability to inhibit the function and produce pathological effects on the stimulated location (24). Therefore, DBS in one area might affect its closest and most interrelated neuron circuits. Thus, inhibiting the PL due to the high-frequency DBS can be followed by induced inhibition in the BLA function. Subsequently, with the suppression of the hyperactivity, acquisition, and persistence of fear avoidance behavior would be greatly reduced in the PL group treated by DBS. In addition, inhibiting the BLA causes some functional changes in the mesocorticolimbic and striatum areas, which can be a reduction in dopamine behaviors such as freezing behavior (1, 27). In this regard, recent experiments have revealed that the freezing behavior in the rat models of post-traumatic stress disorder (PTSD) was well reduced after treatment with high-frequency DBS in both areas. However, considering previous statements, it is predictable that high-frequency DBS in the PL has led to a better reduction in freezing behavior compared to BLA stimulation.
Previous studies have revealed that the acquisition and extinction of the fear memory are associated with the expression of the c-Fos protein in the prefrontal-amygdala loop, in which the PL and BLA are located (28, 29). Therefore, according to the literature, the combination of conditional fear with amygdala DBS might enhance fear extinction and decrease freezing behavior by reducing the expression of c-Fos (1, 22, 30). In fact, the mechanism of the DBS effect may be based on the c-Fos expression in the stimulated area. The c-Fos is considered to be a factor in detecting neuronal activity since its expression is well advanced at the time of action potential (29). In particular, studies have shown the role of c-Fos in the formation and extinction of conditional fear memory as well as the induction effect on the Hypothalamus-pituitary-adrenal (HPA) axis (1, 20, 21). In addition, stressful conditions increase the level of c-Fos protein (26), which leads to the release of corticotropin-releasing hormone (CRH) (20). On the other hand, the hyperactivity of both the PL and BLA causes excessive release of CRH, followed by desensitization of the adrenocorticotropic hormone (ACTH) receptors, which suppresses the release of cortisol/corticosterone from adrenals (21, 22). Moreover, the connection between the PL and the BLA in fear conditioning elevates the CRH resealing. Since CRH plays an important role in the acquisition of fear and memory in emotional trauma, CRH release, induced by c-Fos, causes fear memory acquisition and suppresses fear extinction. It is, in fact, followed by a reduction in the cortisol level (31). According to the outcomes of the current study, high-frequency DBS might reduce hyperactivity in both the PL and the BLA and decrease c-Fos expression and CRH release, which is followed by symptoms relief. On the one hand, by inhibiting the release of CRH, the acquisition of fear, and emotional trauma in the PL and BLA loop is diminished. It facilitates the process of fear extinction and leads to a reduction in avoidance behaviors such as freezing. On the other hand, the inhibition of CRH release is also effective in the release of corticosterone. However, after removing the negative effects of the level of the hormone, it will return to the normal state. According to our findings, DBS treatment in the PL and BLA caused a reduction in both freezing behavior and c-Fos expression and an increase in corticosterone levels. Notably, the results of the DBS treatment in the PL area were superior in the freezing behavior to the BLA group.
The ability to extinguish an emotional response in the face of a no-longer relevant conditional cue is part of a healthy emotional memory system, unlike what happens in some diseases such as PTSD (9, 32). The BLA plays the main role in PTSD conformation. According to the fMRI results, the intensity of PTSD symptoms is directly related to the hyperactivity of the BLA (7, 9). On the other hand, previous studies have shown that in patients with PTSD, c-Fos expression is high due to the elevated activity in different areas such as the BLA (1, 22). Recently, DBS has been clinically used as the treatment for Parkinson disease and psychiatric disorders such as obsessive-compulsive disorder (9). In this regard, previous studies in animal models have revealed that DBS might alleviate PTSD symptoms such as avoidance behavior (1, 7, 9). One recent study showed that DBS in the BLA might alleviate freezing behavior as well as c-Fos expression and normalize corticosterone release in the PTSD animal model using electrical foot shock (1). According to other studies, other areas related to the amygdala might be appropriate as a new way of modulating functions. PL is one of the main areas in the prefrontal cortex in rodents, which plays the main role in the fear acquisition and response as well as the formation of fear memory and conditional fear suppression (20). Moreover, studies not only have shown that there is a similarity between the rodent’s prelimbic (PL) and human’s dorsal anterior cingulate cortex (dACC) but also confirmed that both have the same key role in expressing conditional fear (20, 33). Consequently, due to the connection between the dACC/PL and amygdala, the role of dACC is decisive in enhancing the role of amygdala in the processing of fear memory and suppressing the extinction process. Reducing the activity of both dACC/PL and the BLA in a PTSD patient might improve the patient's behavioral and biological conditions. Additionally, studies have indicated a direct relationship between the sustainability of PL and BLA activities and the freezing behavior, which is a main behavioral symptom in PTSD (6, 8). Therefore, this targeted therapy, using high-frequency DBS, could lead to better therapeutic efficacy and provide a better understanding of the extinction process as an option for PTSD patients.
5.1. Conclusions
The results of the current study demonstrate the potential effect of high-frequency DBS in the PL and BLA regions of the brain on fear extinction in a rodent model. Although similar results were observed after stimulation of both regions, it seems that DBS in the PL is superior to DBS in the BLA for fear extinction applications. This study provided further evidence for the contribution of the prelimbic cortex and amygdala, both in acquisition and extinction processes during contextual fear conditioning. However, the PL stimulation by high-frequency DBS might be more involved in the extinction process and play a more important role as an enhancer.