1. Background
As a major public health problem, violence has diverse negative social consequences. Genetic and neurobiological studies allow realizing the etiology and the development of evidence-based intervention programs to reduce its frequency. Structural brain imaging studies have shown that people with violence and anti-social behavior have changes in gray matter concentration (1). Harmful environments, such as violent treatment, can lead to antisocial outcomes only in a fraction of individuals (2).
Of all the genes that have been attributed to the field of maltreatment, developed authentications have been shown a pivotal role for MAOA X-linked gene (Xp11.23) which locates between DXS14 and OTC (3) and encodes a mitochondrial enzyme entitled monoamine oxidase A. This enzyme plays an important act in the metabolism of neurotransmitters involved in regulating violence, such as serotonin, noradrenaline, and dopamine (4, 5).
This enzyme catalyzes the main brain signaling molecules such as serotonin, noradrenaline, and dopamine and is presumed to indirectly affect antisocial behavior, through the disruption of neurotransmitter balance in brain structures and neural pathways involved in emotional regulation and behavioral inhibition. Any disturbance of enzyme function leads to the accumulation of neurotransmitters, resulting in abnormal mood and behavior (6).
Clinical analyses have approved the function of the MAOA gene in the field of violence. For instance, these lines of studies have demonstrated in mice that insufficiency of this enzyme leads to aggression, abnormal immune response, and defects in processing information (7).
Research in molecular genetics has provided evidence that variations in the MAOA gene have a significant role in the appearance of delinquent behavior. Several studies pointed out a VNTR (variable number tandem repeat) in the upstream of transcription initiation site in monoamine oxidase A gene that is called uVNTR. It associates with the risk of antisocial behavior in adult males committed to criminal activity (8).
The MAOA variants characterize as a VNTR that may contain up to six copies of the 30-bp repeat (9-11). Numerous studies have classified variants using counting numbers as 2R, 3R, and up to 6R (9, 11, 12); but some studies applied a numerical adjustment for the tandem repeats using fractions, i.e., 3.5R, 4.5R, and 5.5R (13-15).
Alleles with 2, 3, 5, or 6 copies have a low-efficiency transcription. Subjects harboring these “low-activity” MAOA alleles (MAOA-L) are more probably to demonstrate antisocial personality signs as compared to persons who have “high-activity” MAOA alleles (MAOA-H), i.e., alleles with 3.5 or 4 copies (16).
Since no genetic polymorphisms have been considered to have a definite influence on human behavior, the capability to forecast whether a person is likely to perpetrate a criminal act in regard to their genetic profile is an idealistic strategy. However, recent attempts to predict the individual's vulnerability to violence based on his/her genetic makeup has attracted great curiosity both from a scientific attitude as well as implications for criminology and rehabilitation strategies (17).
2. Objectives
The purpose of the current study was to make inquiries about the association between MAOA uVNTR polymorphism and antisocial behavior in a population of Iranian incarcerated adult males in central Iran.
3. Methods
3.1. Subjects
This study included 183 adult males consisting of 88 violent subjects referred to Isfahan Legal Medicine Organization in central Iran and imprisoned in a large city prison in the central Iran and 95 age-matched non-violent individuals (controls) without any history/sign of antisocial behavior. Participants ranged in age from 18 to 60 years. Anti-sociality had been evaluated by the aid of a diversity of behavioral and psychological measures comprising an evaluation of antisocial personality disorder and a tendency to violence and infarction (18).
Case participants were incarcerated men who were diagnosed with DSM-IV criteria as ASPD (Antisocial personality disorder). Control subjects were healthy volunteers with no former background of psychiatric illness or substance abuse based on a SCID I assessment for DSM-IV criteria and no cluster B category personality disorder based on a SCID II assessment (19, 20). All individuals were further checked for mental status and schizophrenia by licensed psychiatrists of the Isfahan Legal Medicine Organization.
The current study was only executed on male convicts because of the proved relationship between MAOA-L variants and aggression in men, but it has not been observed in women (5, 21, 22).
Blood specimens were collected in EDTA-treated tubes and stored at –20°C for genetic analyses. Informed consent was collected before the participation, and all experimental steps were endorsed by the Ethics Committees of Shahrekord University and Isfahan University of Medical Sciences.
3.2. DNA Extraction and Genotyping
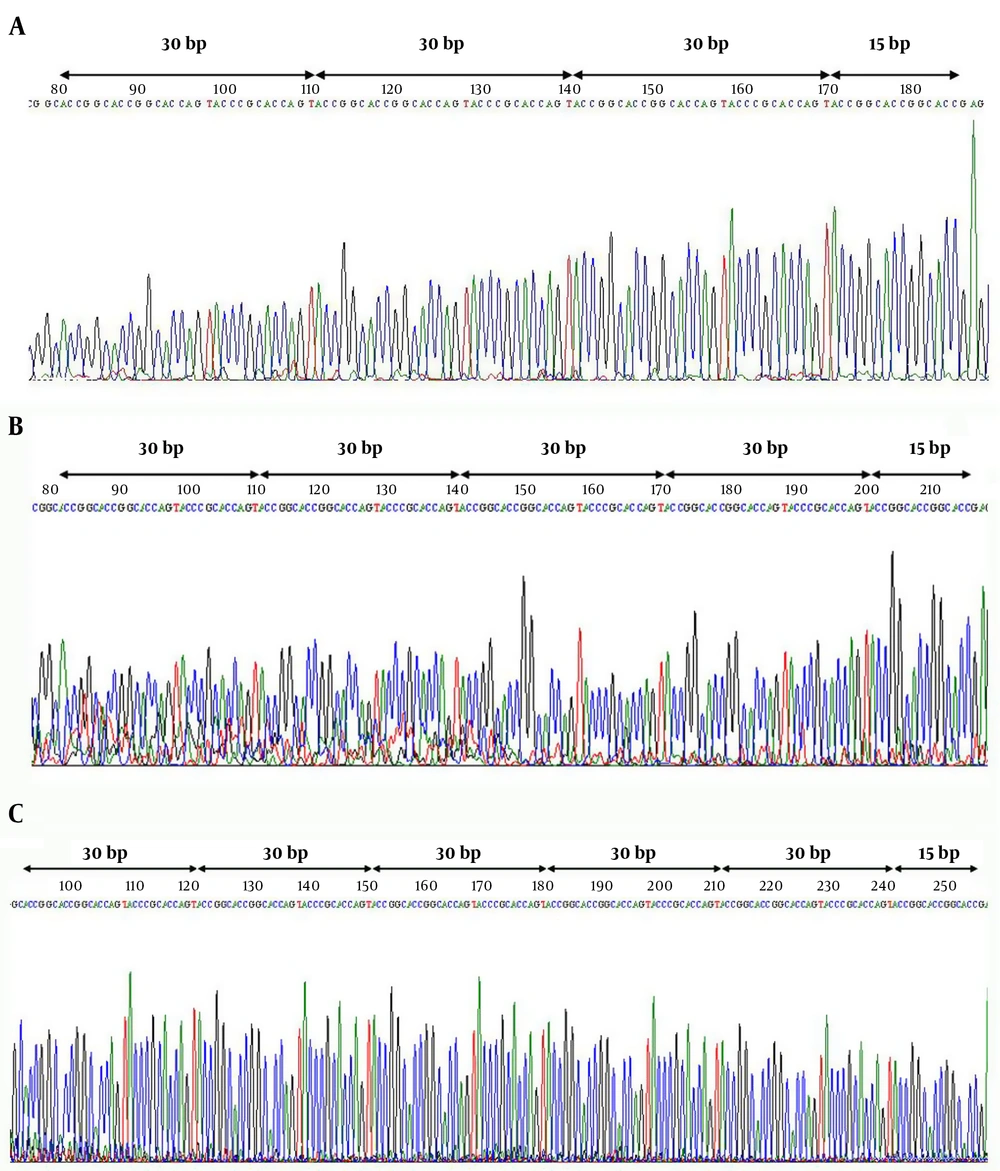
DNA was isolated from blood samples, by the use of a DIAtom DNA extraction kit (Isogen Laboratory, Russia). The quality and quantity of the extracted DNA were assessed by agarose gel electrophoresis and spectrophotometry. The MAOA uVNTR allelic polymorphisms were genotyped by using PCR-based amplification, with the following primers: forward, 5’ACAGCCTGACCGTGGAGAAG- 3’; and reverse, 5’CAGGCTGTAGGAGGTGTCGTC. The PCR reactions included 1 µL of template DNA, 12.5 µL of PCR Master Mix (Ampliqon, Denmark), 0.5 µL of each primer (10 µM) in a total volume of 25 µL. Thermal cycling conditions for amplifying the MAOA uVNTR amplicon were as follows: 95°C for 5 min for the initial denaturation followed by 35 cycles consisting of denaturation at 94°C for 30 sec; annealing at 65°C for 30 sec; extension at 72°C for 40 sec and the last extension at 72°C for 5 min. The PCR products were run on 10% polyacrylamide gel visualized by silver staining. Moreover, to identify the types of alleles, some of the PCR products were Sanger sequenced (Macrogen, Seoul, Korea).
3.3. Statistical Analysis
In this study, to examine the relationship between the MAOA 30-bp VNTR variants and the antisocial behavior, three-step analytical processes were pursued. The initial step was determining the number of 30-bp repeats in the MAOA gene in cases and controls. In the second step, the genotype and allelic frequencies of each individual was calculated. Finally, to investigate the MAOA allelic dispensation between case and control groups, a chi-square test was performed using SPSS version 16 (SPSS Inc, Chicago, IL). A P value of less than 0.05 was considered statistically significant.
4. Results
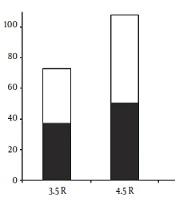
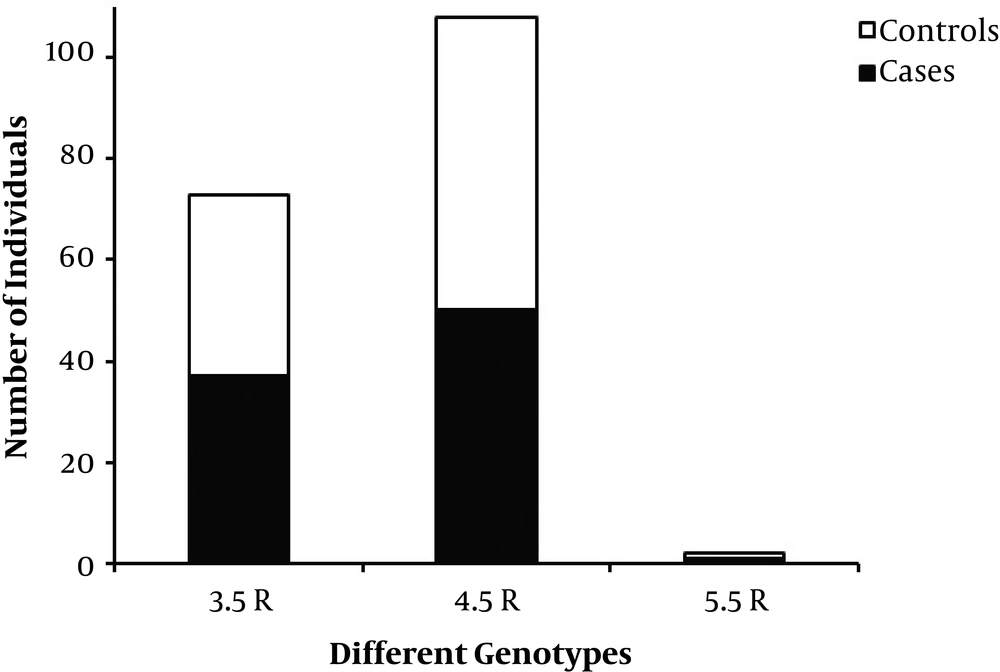
After PCR optimization, genotypes of case and control samples were determined by loading on 10% polyacrylamide gel and direct PCR-sequencing. We identified three MAOA uVNTR allelic variants harboring fraction numbers of the repeat; including 3.5, 4.5, and 5.5 variants. Furthermore, the most common and the rarest observed alleles in both groups were 4.5R and 5.5R, respectively (Table 1). Of note, alleles containing counting numbers of the repeat i.e. 2, 3, 4, 5, and 6 variants were not observed in any of the two groups. These results were confirmed by several rounds of direct PCR-sequencing (Figure 1). It should be noted that as the MAOA gene is situated on the X chromosome, men are consequently hemizygous for the MAOA gene. Figure 2 displays the frequencies of genotypes and alleles in case and control groups. The association between antisocial behavior and MAOA gene allele variants was assessed using the chi-square test. Results did not show a statistically significant association between antisocial behavior and allele frequencies in the studied population (Table 1).
| Group | Total (N) | Allele/Genotype Frequencies (Percentages) | χ2 | P Value | ||
|---|---|---|---|---|---|---|
| 3.5 R | 4.5 R | 5.5 R | ||||
| Controls | 95 | 36 (37.9) | 58 (61.05) | 1 (1.05) | 0.339 | 0.844 |
| Cases | 88 | 37 (42.04) | 50 (56.82) | 1 (1.14) | ||
5. Discussion
Examining the plausible association between genetic variation in MAOA gene and behavioral characteristics correlated with aggression has been described in dozens of studies (8, 23-30). However, due to the differences in genetic profiles and allelic frequencies of the MAOA uVNTR in various populations, studies are yet essential to explore if there are some correlations in various racial/ethnic groups (31).
In the current study, we identified three repeat variants harboring fraction numbers of the repeat, including 3.5R, 4.5R, and 5.5R. The frequency of the rarest 5.5 copy variant was 1.05% in healthy controls and 1.14% in antisocial subjects. However, the 4.5 repeat variant was very common in the studied population: 61.05% in controls and 56.82% in cases. In 1998, Sabol et al. (9) genotyped uVNTR polymorphism in the MAOA promoter in a total of 2156 individuals. Alleles with 4 and 3 repeats were the most common forms in all populations studied with the frequencies of 62% and 36%, respectively, while alleles with 3.5 and 5 repeats were always rare. In a study by Huang et al. in 2004 (11), a novel and rare repeat variation with six repeats was identified. An additional rare allele with 2 repeats was detected in an Italian population. Another rare allele (3a allele) which contained 3 repeats plus 18-bp (CCAGTACCGGCACCGGCA) of the repeated motif, was also reported (10). Although the two most common alleles are those with 4 and 3 repeats (18), this observation was not confirmed in our samples. However, regarding the recent study by Im et al., and using the same assumption of theirs, it may state that the observed allele frequencies in the sample of the Iranian population are more similar to Caucasians than east Asians, including Chinese, Japanese and Korean individuals. As a result, we demonstrated the changes in allele frequencies in MAOA uVNTR by race and geographic location (9, 32-36). Furthermore, whether the length of the uVNTR variants affects the promoter activity of the MAOA gene is not fully elucidated. Various studies using in vitro systems are required to determine the effect of the uVNTR variants on expression levels of MAOA. Regarding these studies, there are clear inconsistencies in the promoter activities of the uVNTR variants. Overall, the inconsistencies may be assigned to two factors: the exerted MAOA-luciferase reporter gene constructs and the included length of the promoter (35, 37-39).
In the current study, we could not detect a remarkable relationship between different uVNTR polymorphisms in the MAOA promoter and antisocial behavior. Caspi et al. reported the first evidence in a study (from birth to maturity) on a large sample of abused children with low-activity alleles of MAOA which were more probable to make antisocial issues in adulthood as the comparison to abused children with the MAOA-H variants (8). Williams et al. reported a significant MAOA genotype effect for the antisocial index because of higher antisocial traits in MAOA low-activity alleles relative to MAOA high-activity ones (40). The MAOA-L genotype was also correlated with violent behavior in the crime cohort in Finland (41). In contrast to other studies, Beitchman et al. reported a significant correlation between MAOA uVNTR alleles with 4 repeats (a high-activity MAOA variant) and aggressive behavior (42). In a study by Widom et al. (34), no remarkable effect was discovered for the association between MAOA genotype and antisocial behavior. Prichard et al. also reported no significant relationship between uVNTR variants in the MAOA promoter with antisocial behavior in Caucasian men and women (43). Taken together, our result on observing no association between the uVNTR polymorphism in the MAOA gene and antisocial behavior is consistent with results reported by Widom et al. (34) and Prichard et al. (43), but in contrast to others (8, 40). Therefore, the discrepancy observed between our data and the others may be related to the populational characteristics.
To sum up, Iranian individuals in central Iran may have a distinctive profile of the uVNTR polymorphisms in the MAOA gene. A pivotal question is how much of these MAOA uVNTR polymorphisms, we identified in the current study, may influence the MAOA activity and brain morphology and function in the behavioral characteristics correlated with aggression. Furthermore, our study calls for further population-based study in the Iranian population, including longitudinal studies in the normal population.