1. Background
Multiple sclerosis (MS) is the most common immune-mediated inflammatory demyelinating disease of the central nervous system (CNS) with a prevalence of 30 per 100,000 (with a range of 5 - 80) worldwide (1). Magnetic resonance imaging (MRI) is an important diagnostic tool for MS because it produces clear images of pathological changes in lesions in the brain and spinal cord of MS patients. Thus, it could be useful to monitor the statuses of disease and how the patient responds to treatment (2). MRI has improved the diagnostic work-up in monitoring statuses of MS patients, but inappropriate conventional techniques could effect on detection of different kinds of lesions; for example, some MRI artifacts in T2-fluid-attenuated inversion recovery (FLAIR) images could be considered as brain lesions or patient’s small movement changes the detection of the lesions. Also, subtle changes at the onset of brain tissue damage have not been seen in conventional images. Therefore, appropriate protocols, like dynamic contrast-enhanced (DCE)-MRI, can detect dysfunction of blood-brain-barrier (BBB), which occurs in the early stages of the inflammatory cascade process and are beneficial in the early stages of newly forming lesions that are not seen in conventional protocols, like T1 or T2-FLAIR. Improvements in MRI techniques and the use of appropriate MRI protocols based on variation in the brain during MS can be promising for a better definition of the typical parameters of MS lesions (3).
MS-related lesions can be appeared on MRI images as either bright or dark spots, depending on the type of MRI protocols used. In T2-weighted MRI protocol is applied commonly to quantify the lesion load and T1-weighted to differentiate edema, blood-brain barrier (BBB) breakdown by contrast enhancement, and irreversible brain tissue damage (black holes) (4). Pathologically, in different kinds of MS lesions, active enhanced lesions hallmark damages BBB, which leads to the infiltration of the inflammatory cells into the CNS. Thus, the inflammatory demyelination and vasogenic edema present in early MS lesions and active lesions. Chronic lesions are more frequent in progressive MS patients and are characterized by a slowly expanding rim of activated brain immune cells (microglia) (5). Perivascular inflammatory infiltrates are often encountered in chronic lesions. As the lesions progress from acute active to chronic lesions, edema resolves, inflammation decreases, and brain immune cells gradually disappear. Accordingly, axonal damage and loss are also observed in chronic lesions. T1-black holes may represent either area of edema or axonal loss and severe tissue destruction, which are a marker of neuronal loss and neurodegeneration in brain tissue and measure disease activity in progressive MS patients (6). T1 black holes result from an expansion of the extracellular space due to an increase in water content or deterioration of structural components (7). Conventional MRI (CMRI) is a useful prognostic tool to obtain valuable information about the number, location, and inflammatory activity of MS-related lesions, but it provides limited sensitivity to pathology alteration in the cortex and principle of focal damage. Thus, the advanced modality of MRI could give us an insight into focal damage and pathology of different kinds of lesions, such as axonal and myelin damage (8-12). DCE-MRI gives information about subtle BBB alteration in lesions, which help us to quantify immunity cells infiltration and activity of lesions. Perfusion parameters, like cerebral blood flow and cerebral blood volume, are proper markers to investigate the amount of delivered blood to different kinds of MS lesions and hemodynamic changes in the brain and could be a clue to the presence of inflammation in different kinds of MS lesions (13). Also, magnetization transfer imaging is a good modality to quantify vasogenic edema and abnormality in water content or damaged structure of brain tissue, which are observed in chronic MS lesions (14). Therefore, a combination of imaging modalities could provide quantitative information to distinguish active, chronic, and black hole lesions (15).
2. Objectives
In this study, multi-parametric quantitative MRI modalities were used to investigate the amount of inflammation-related alterations in different kinds of MS lesions to obtain the following objectives: (A) introduction of a hallmark biomarker to differentiate between the types of lesions; (B) introduction of a multi-parametric MRI protocol to use in clinical MRI to detect subtle alterations caused by inflammation in active and chronic lesions.
3. Methods
3.1. Subjects
Images of 18 patients with a clinical diagnosis of relapse-remitted multiple sclerosis (RRMS) using MacDonald Criteria (16), who were admitted at MS Clinic of the Research Institute of Neurology in Imam Hospital in Tehran, Iran, were obtained with conventional and DCE-MRI and magnetization transfer imaging protocols. Table 1 shows the details of the patient’s demographic descriptions. MRI, according to a standard clinical MS protocol with the addition of multi-parametric MRI before and after administration of a gadolinium-based contrast agent, was performed. The local institutional review board approved the study, and written informed consent was obtained from all patients.
| MS Patients | ||
|---|---|---|
| Female | Male | |
| Number of patients | 6 | 12 |
| Median age, y | 43 | 31 |
| Age range, y | 31 - 50 | 27 - 62 |
| Disease duration, y | 2 - 10 | 5 - 15 |
| EDSS | 0 - 4 | 1 - 5 |
| The last replace before inclusion, week | 2 - 6 | 2 - 6 |
Demographic Characteristics and Clinical Description of the Disease Stage of Multiple Sclerosis (MS) Patients
3.2. MRI imaging Acquisition
Images were acquired on a 3 Tesla MRI scanner (DISCOVERY MR750w: GE Healthcare, Chicago, Illinois, United States) using a 24-channel phased-array head coil. The main acquisition parameters of the sequence are presented in Table 2.
| Sequence | Acquisition Parameters | Values |
|---|---|---|
| DCE sequence | AX 3D-SPGR | |
| TR | 3.34 ms | |
| TE | 1.28 ms | |
| FOV | 300 × 300 mm2 | |
| Slice number | 900 | |
| Thickness | 5 | |
| MT sequence | AX 3D-SPGR | |
| TR | 28 ms | |
| TE | 5 ms | |
| FOV | 240 × 240 mm2 | |
| Slice number | 64 | |
| Thickness | 2 | |
| MT pulse | Fermi, 2400 Hz | |
| Pre- and post-contrast T1-weighted sequence | AX 2D-SE | |
| TR | 6000 ms | |
| TE | 11 ms | |
| FOV | 230 × 230 mm2 | |
| Slice number | 23 | |
| Thickness | 5.5 | |
| FALIR | AX, COR 2D-FSE | |
| TR | 8000 | |
| TE | 119.88 | |
| FOV | 230 × 230 mm2 | |
| Slice number | 23 | |
| Thickness | 5.5 |
Main Acquisition Parameters of the Magnetic Resonance Imaging (MRI) Sequences Used for Imaging
3.2.1. Image Analysis
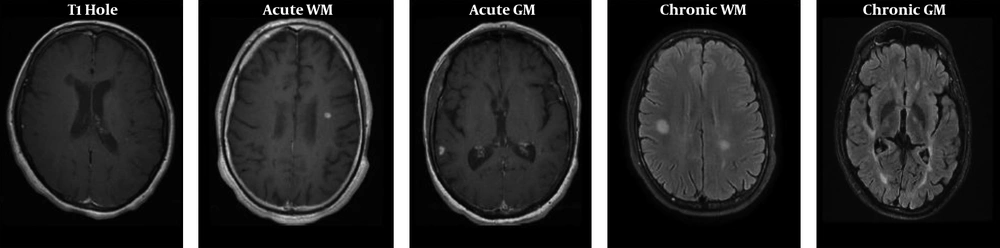
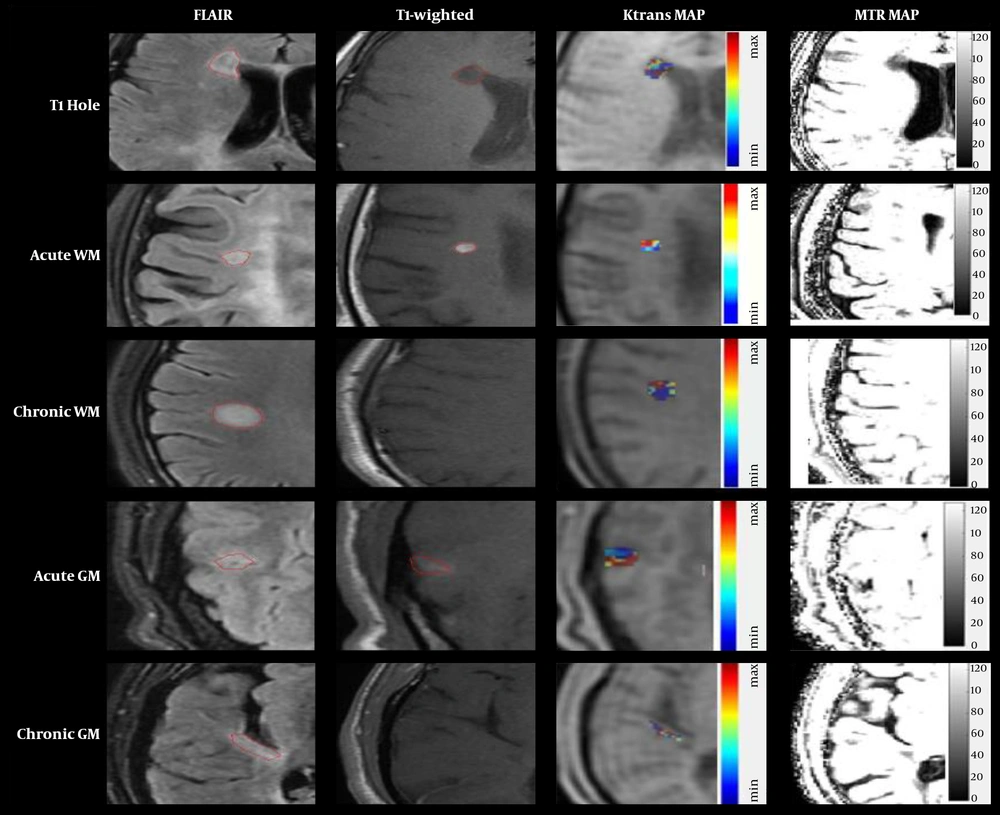
To assure that the injection rates and patients’ cardiovascular states do not affect the kinetic parameters, the arterial input function (AIF) was incorporated into the two-compartment model. Here, the AIF corrected the kinetic analysis by measuring the signal from the regions of interest (ROI) in the middle cerebral artery on T1-weighted images and adjusting the results accordingly. Other RIOs located in T1 hole, acute white matter (WM), chronic WM, acute gray matter (GM), and chronic GM (Table 2 and Figure 1) indicate different lesions in T2-FLAIR and post-contrast T1-weighted images. ROIs were outlined manually by an experienced radiologist. First, in post-contrast T1 ROI was manually placed on the enhancement of active lesions. Then, ROIs were masked and placed on T2-FLAIR images in MRI-CRO (Nottingham, UK) because FLAIR images had the best contrast to determine MS lesions. In chronic lesions without any gadolinium (Gd)-enhancement, ROIs manually were drawn on selected lesions in T2-FLAIR images. Finally, ROIs were placed on perfusion-weighted imaging (PWI) images. The magnitude of enhancement varied across the lesion. Acute active lesions in both WM and GM were determined by comparing pre- and post-contrast T1-weighted images, and all of the T1-weighted images were compared with T2-FLAIR because of the high detectability of different kinds of lesions in this sequence. Recognized chronic lesions from T2-FLAIR images were compared with post-contrast T1-weighted images to ensure that there was no Gd uptake. Also, obtained images from 3D-SPGR protocol in magnetization transfer imaging (MTI) could be beneficial in cortical GM lesions. The supervised assistant neurologist confirmed all detected lesions. The obtained ROIs of perfusion-weighted images were then analyzed using the DCE Tool plug-in (version 2.0SP1) within ClearCanvas (Toronto, Ontario, Canada) framework. The DCE Tool provides options to model contrast uptake within an ROI using the modified Toft’s model (17). The DCE Tool computed these metrics for each ROI. The most important physiologic parameter of the tissue is Ktrans, which demonstrates a combination of both tissue perfusion and permeability. Also, in-house developed MATLAB code was used to obtain cerebral blood volume (CBV) and cerebral blood flow (CBF) of the mentioned lesions measured by perfusion parameters in the DCE-MRI sequence. In MT and non-MT data, RIOs of each kind of lesions were drawn manually in MRI-CRO software, and signal intensity in both MT and non-MT images were measured. The MTR parameter was measured using the following Equation 1:
Where M0 is the value of the signal in non-MT images and Ms is the value of the signal in MT images.
3.3. Statistical Analysis
Each of the four parameters was assessed (both mean and SD of MTR, Ktrans, CBV, and CBF) in different kinds of lesions (T1 holes, acute and chronic WM, and acute and chronic GM). A multi-parametric test (ANOVA) was used to detect significant differences using SPSS version 21.0 software (SPSS Inc., Chicago, IL, USA). Finally, a multivariate analysis using support vector machines (SVMs) was performed using Weka3 (University of Waikato). This classifier showed the best parameter to show differences between lesions.
4. Results
Five groups of lesions in T2-FLAIR and post-contrast scans are displayed in Figure 1. Also, the Ktrans and MTR maps in marked lesions are represented in Figure 2.
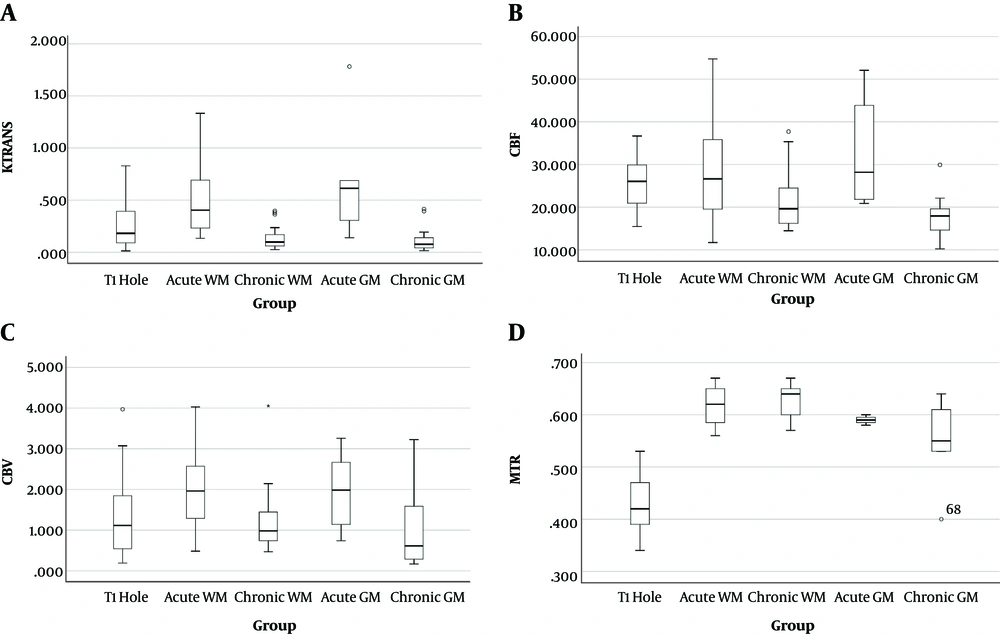
Analysis of four parameters of Ktrans, CBF, CBV, and MTR in five groups of lesions showed significant differences between each parameter in different kinds of MS lesions, which are represented in Table 3. The Ktrans mean value of acute GM lesions was the highest value among the five groups of lesions. Except for CBV, other parameters showed similar results to Ktrans. As shown in Table 3, the p-values of all measured parameters obtained by the ANOVA were less than 0.05, thus, these parameters had significant differences. The values of the parameters of Ktrans, CBV, CBF, and MTR are displayed in Figure 3, respectively.
| Numbers | Values | ANOVA; P Value | |
|---|---|---|---|
| KTRANS | < 0.001 | ||
| T1 hole | 20 | 0.26 ± 0.22 | |
| Acute WM | 20 | 0.51 ± 0.34 | |
| Chronic WM | 80 | 0.14 ± 0.12 | |
| Acute GM | 6 | 0.69 ± 0.58 | |
| Chronic GM | 24 | 0.13 ± 0.13 | |
| CBV | 0.048 | ||
| T1 hole | 20 | 1.37 ± 1.05 | |
| Acute WM | 20 | 1.98 ± 0.98 | |
| Chronic WM | 80 | 1.25 ± 0.82 | |
| Acute GM | 6 | 1.96 ± 0.94 | |
| Chronic GM | 24 | 1.11 ± 1.04 | |
| CBF | < 0.001 | ||
| T1 hole | 20 | 25.75 ± 6.27 | |
| Acute WM | 20 | 29.52 ± 12.14 | |
| Chronic WM | 80 | 21.45 ± 6.71 | |
| Acute GM | 6 | 32.49 ± 12.67 | |
| Chronic GM | 24 | 17.53 ± 5.37 | |
| MTR | < 0.001 | ||
| T1 hole | 20 | 0.43 ± 0.07 | |
| Acute WM | 20 | 0.62 ± 0.04 | |
| Chronic WM | 80 | 0.63 ± 0.03 | |
| Acute GM | 6 | 0.59 ± 0.01 | |
| Chronic GM | 24 | 0.55 ± 0.09 |
The Analysis of Variance (ANOVA) Results of the Four Parameters (Ktrans, CBV, CBF, and MTR) in Five Kinds of T1 Hole, Acute WM, Chronic WM, acute GM, and Chronic GMa
A, Comparison of the Ktrans; B, cerebral blood flow (CBF); C, cerebral blood volume (CBV); D, and magnetization transfer ratio (MTR) parameters in various lesions. Box plots represent the median value (50th percentile) and the range of density of the lesions. Outliers marked with a circle are mean values of each parameter in certain lesions.
To investigate differences between two groups of acute and chronic lesions in WM and GM lesions, a t-test was applied, and the results for WM acute and chronic lesions showed significant differences between Ktrans, CBV, and CBF (P < 0.05) (Table 4). Regarding acute and chronic lesions in GM, only CBF had a significant difference (P < 0.05) between the two groups (Table 5).
| Group | Values | P Value |
|---|---|---|
| KTRANS | < 0.001 | |
| Acute WM | 0.51 ± 0.34 | |
| Chronic WM | 0.14 ± 0.12 | |
| CBV | 0.014 | |
| Acute WM | 1.98 ± 0.98 | |
| Chronic WM | 1.25 ± 0.82 | |
| CBF | 0.013 | |
| Acute WM | 29.52 ± 12.14 | |
| Chronic WM | 21.45 ± 6.71 | |
| MTR | 0.577 | |
| Acute WM | 0.62 ± 0.04 | |
| Chronic WM | 0.63 ± 0.03 |
The t-test Results Comparing Four Parameters of Ktans, CBV, CBF and MTR in Acute and Chronic WM Lesionsa
| Group | Values | P Value |
|---|---|---|
| KTRANS | 0.063 | |
| Acute GM | 0.69 ± 0.58 | |
| Chronic GM | 0.13 ± 0.13 | |
| CBV | 0.107 | |
| Acute GM | 1.96 ± 0.94 | |
| Chronic GM | 1.11 ± 1.04 | |
| CBF | 0.033 | |
| Acute GM | 32.49 ± 12.67 | |
| Chronic GM | 17.53 ± 5.37 | |
| MTR | 0.383 | |
| Acute GM | 0.59 ± 0.01 | |
| Chronic GM | 0.55 ± 0.09 |
The t-test Results Comparing Four Parameters of Ktans, CBV, CBF, and MTR in Acute and Chronic GM Lesionsa
The SVM was used to indicate the best parameter between four parameters in different types of lesions, which measured the mean value of parameters to introduce appropriate biomarkers to demonstrate different characteristics of the lesions. The results indicated the feature weights, respectively, as follows: MTR, CBV, CBF, and Ktrans (Table 6). According to the measured SVM classifier rank weights, MTR was the best biomarker to distinguish between five kinds of lesions.
| Parameter Rank | Parameter | SVM Weight |
|---|---|---|
| 1 | MTR | 1.074 |
| 2 | CBV | 0.527 |
| 3 | CBF | 0.326 |
| 4 | KTRANS | 0.092 |
The Results of the Comparison of Lesions Using SVM Classifier to Determine the Best Parameter to Distinguish Different Kinds of Lesions
5. Discussion
The aim of this study was to assess whether non-conventional multi-parametric quantitative MRI can be used to detect the pathological change in various types of MS-related lesions changes, like subtle BBB dysregulation and microstructural variations that cannot be revealed by conventional protocols. Therefore, the development of a proper method to differentiate between different kinds of lesions that have not yet been investigated is beneficial to demonstrate pathological changes during different stages of MS. In this study, four quantitative parameters were investigated in different types of lesions in MS patients. Also, this study assessed alterations based on different aspects of inflammation in various MS-related lesions reviewed earlier by researchers. Previous studies have proved that MS acute lesions massively were contributed to perivascular and parenchymal inflammatory infiltrate and also have demonstrated that inflammation was the reason for demyelination and axonal degeneration, which can be observed in chronic and black holes lesions (5, 18). According to the remarkable ability of MRI modalities to detect subtle changes in the brain, DCE-MRI had the potential to detect alteration caused by inflammation. Applied methods in this research could reveal alterations that happened during inflammatory processes in the brain (19), and multi-parametric MRI could show inflammatory-related changes by an observed abnormality in T1, T2, and T2* relaxation time and MT ratio (20). Dynamic imaging of the hemodynamic changes during inflammatory diseases, like MS, has shown abnormality in cerebral permeability, which can indicate dysfunction BBB in MS brains compared with normal volunteers (21). In agreement with the results of other studies, measured perfusion parameters, like Ktrans, CBV, and CBF in various lesions had significant differences between chronic and active lesions, which indicates that their values changed differentially in all lesions and could be a proper biomarker to detect the number of perfusion changes in MS-related lesions. A high value of Ktrans, CBV, and CBF has been observed in acute lesions related to structural changes during inflammation. The low value of parameters in chronic lesions, including black holes, has been shown due to neurodegeneration in these lesions, which is associated with neuronal and axonal loss (22). Until now, there is no study on all lesions in MS brains; however, several studies have reported a reduction in CBV and CBF in MS lesions, which had a significant difference between normal white and GM (23). Cortical GM damage is now widely recognized in MS. The standard MRI does not reliably detect cortical GM lesions. Therefore, advanced protocols, like 3D-spoiled gradient recalled echo that is used in MT imaging, could reliably and quantitatively assess cortical GM damages, like active or chronic lesions in MS patients, as our results showed (24).
Measurements of perfusion characteristics in MS lesions compared with the normal-appearing WM have been shown that BBB interruption has an important role in enhancement and uptake of Gd-based contrast agents and could demonstrate inflammatory activation in acute MS lesions. Also, DCE-MRI has a high sensitivity to reveal dysfunction of BBB earlier than routine protocols, such as post-contrast T1-weighted images (25). Evaluations of the black holes have confirmed the results of other investigators, indicating the presence of edema and axonal loss in T1 holes with a higher value of quantitative parameters than chronic lesions associated with axonal loss and neuronal degeneration (6, 22, 26). Magnetization transfer imaging investigations on MS patients have been recently considered, and this modality could show microstructural changes during the formation of MS lesions. The presence of inflammation and vasogenic edema in MS-related lesions can cause an increase in the water content of brain tissue (27). Measurements of the microstructure of lesions in long-term evolution in normal-appearing WM and MS lesions have shown a significant decrease in this parameter that is contributed to an abnormality in MS lesions, which is consistent with our results. Non-conventional MRI investigations on MS lesions have shown the relevance between MTR and myelin content of lesions, which has been shown by a comparison between histopathological analysis and MTI. They have shown a greater pathologic specificity compared with standard protocols that were confirmed by our results (28-30). Our ANOVA results showed that MTR also changed significantly in different kinds of lesions, which was related to the pathology of the lesions (5, 22). A short-lived inflammatory process has been observed in acute MS lesions. The pathology of lesions has demonstrated that the content of myelin in black holes had the lowest value in MTR because of the persistence effect of neuronal and axonal loss. Also, destroyed myelin in chronic lesions is more than active lesions because of the presence of edema and microglia activation in acute lesions. Accordingly, measurements have shown that the MTR had a lower value in chronic lesions (31, 32).
SVM classifier analysis was performed to define the best parameter, which showed the best rank weights between measured parameters in all kinds of lesions. Due to the differences in measured parameters, MTR was indicated as the best parameter to show the difference between normal WM and MS lesions (20). Also, this research introduced MTR as the best biomarker to indicate significant differences between different types of lesions, and MTR was an appropriate parameter to differentiate between various types of MS-related lesions.
5.1. Conclusions
Recently, impressive progress has been made in the investigation of several aspects of advanced MRI techniques, which has provided a detailed and new outlook into the pathological-related alterations in brain tissue during MS. Histopathological and imaging methods have indicated that inflammatory and neurodegenerative phenomena have important roles in MS lesions pathology. This study assessed appropriate parameters, like MTR and perfusion parameters, to describe pathology alterations in lesions. MTR changes may reveal the formation of acute active MS lesions several months before observation by conventional protocols. Thus, MTI is a modality that could reveal processes involved in the formation of active lesions. Also, DCE-MRI with high sensitivity to subtle changes in BBB could detect early changes in the formation of active lesions. Thus, the proposed protocols could be useful for early detection of newly forming lesions before T1 or T2-FLAIR images as well as for earlier diagnosis of the disease stage and save the time to begin treatment. This research tried to evaluate quantitative MRI methods to distinguish different kinds of MS-related lesions, and our results introduced MRI parameters to indicate structural and pathological differences in the lesions, which were related to the inflammatory and neurodegenerative process. Finally, one of the measured parameters was introduced as the best parameter to indicate differences in lesions associated with water and myelin content of brain tissue.
In summary, multi-parametric MRI could assess and determine changes in lesions that cannot be provided by conventional and clinical routine MRI. Thus, the mentioned modality can help to reveal subtle changes in MS lesions with different microstructural and pathological changes related to inflammation.
.jpg)