1. Background
Parkinson's disease (PD) is a progressive neurodegenerative disorder that affects 0.3% of the global population over 40 years of age. About 7.5 million people worldwide and one million people in the North America suffer from it (1-6).
In the early stages, an area of the brain called the substantia nigra progressively involves in the disease and increases over time (7). The neurological manifestations of the disease seem to be due to the alteration of inhibition and stimulation patterns in the basal ganglia and their association with direct and indirect pathways in this area. Dopamine and acetylcholine act as neurotransmitters in this area. In idiopathic Parkinsonism, the balance of these two substances that interact with each other is impaired, and dopamine depletion occurs in the nigrostriatal system (8).
There are numerous environmental factors contributing to PD etiology, including toxic metals. Amongst all, lead (Pb) has shown a neurotoxic effect via passing over the blood-cerebrospinal fluid barrier (BCB), accumulating in choroid plexus (CP), disrupting the prefrontal cerebral cortex, hippocampus, and cerebellum leading to several neurodegenerative disorders (9, 10). The neurotoxic potential of Pb is due to the interference with several neurotransmission systems, including those of dopaminergic, cholinergic, and glutamatergic (11). According to occupational exposure data, the risk of PD increases along with whole-body lifetime exposure to lead (12). It has also been reported that cumulative lead exposure can significantly increase the risk of PD (13). A recent meta-analysis reported that exposure to lead is involved with 50% increased risk of PD (14).
Cadmium (Cd) is another neurotoxic metal associated with various neurological diseases. Cadmium is known to induce oxidative stress as a result of inhibiting the activity of acetylcholinesterase enzyme along with the depletion of glutathione, superoxide dismutase, and other antioxidants in central nervous system (CNS) (15). The association of Cd exposure with risk of developing several neurological symptoms, including parkinsonism has been reported (16). This element can cause some neurological disorders through neuronal apoptosis, damage to the neural pathways, including the mammalian target of rapamycin (mTOR), and the production of the reactive oxygen species (17).
2. Objectives
If we can find a relation between the serum levels of these metals and PD, it can be used as one of the most preventable factors in the development of this disease. Hence, the present study aimed to determine the serum lead and cadmium levels in Parkinson's patients and compare them with those of the healthy controls.
3. Methods
This case-control study was conducted on PD patients and healthy controls during 2017 - 2018 in our university hospital. A total of 100 PD patients and 30 healthy controls were randomly assigned into two groups. The demographic information (age, sex, education, place of residence, marital status, and smoking) and clinical data (duration of the disease, stages of the disease, severity of disease, type of medication, and duration of use) were collected from the patients. About 5 mL of peripheral blood was taken from each person, and plasma was separated immediately, then centrifuged and stored at -80ºC until further analysis.
3.1. Measuring the Serum Levels of Lead and Cadmium by Atomic Absorption Spectrophotometry
Atomic absorption spectrophotometry (AAS) was used to measure cadmium and lead levels. This device is used for measuring heavy metals using an atomic absorption of the light rays, which is used to determine the concentration of an element in a sample. The absorbed wavelength is different for each element and is specific to the element that directly depends on the concentration of the light absorbing atoms (the concentration of the element).
The thermal cycles used for measuring the cadmium levels are presented in Table 1. Also, the device's setting for measuring the serum levels of cadmium and the thermal cycle of the device was as follows:
- Analytical line: 228.8 nm; Bandwidth, 0.4 nm; Filter factor, 0.1; Lamp current, 5.0 ma; Integration time, 3.0 sec; Background, D2; Graphic type, platform coated; and Sample size, 10 µL.
- Acidity: 0.1% nitric acid; Sensitivity, 0.4 pg/mL; Detection limit, 0.25 pg/mL; and Working range, 0.10 - 6 ng/mL.
| Stage | Temp (°C) | Ramp (s) | Hold (s) | Gas |
|---|---|---|---|---|
| 1 | 90 | 5 | 10 | High |
| 2 | 120 | 5 | 10 | High |
| 3 | 500 | 5 | 10 | High |
| 4 | 1800 | 0 | 3 | Off |
| 5 | 1900 | 1 | 2 | High |
To make a standard solution, 10 μL of cadmium nitrate solution at the concentration of 1000 ppm was diluted to 1 cc of 0.1% nitric acid. Using this solution, concentrations of 10 ppm, 100 ppb, 10 ppb, 5 ppb, 2.5 ppb, 1.25 ppb, and 0.625 ppb were prepared by serial dilutions, and 0.1% nitric acid solution was used for dilution.
The thermal cycles that used for measuring the lead levels are presented in Table 2. Also, the device's normal setting for measuring the serum levels of lead and the thermal cycle of the device was as follows:
- Analytical line: 283.3 nm; Bandwidth, 0.4 nm; Filter factor, 0.1; Lamp current, 3.0 ma; Integration time, 3.0 sec; Background, none; Graphic type, platform coated; and Sample size, 10 µL.
- Acidity: 0.1% nitric; Sensitivity, 5.18 pg/mL; Detection limit, 3.88 pg/mL; and Working range, 1 - 100 ng/mL.
| Stage | Temp (°C) | Ramp (s) | Hold (s) | Gas |
|---|---|---|---|---|
| 1 | 70 | 10 | 10 | High |
| 2 | 110 | 10 | 10 | High |
| 3 | 450 | 10 | 15 | High |
| 4 | 1800 | 0 | 2 | Off |
| 5 | 1900 | 1 | 2 | High |
To make the standard solution, 10 μL of lead nitrate solution at a concentration of 1000 ppm was diluted to 1 cc of 0.1% triton. Using this solution, concentrations of 10 ppm, 100 ppb, 50 ppb, 25 ppb, 12 ppb, 6 ppm, and 3 ppb were prepared by serial dilutions, and triton 0.1% solution was used for dilution.
3.2. Inclusion Criteria
The inclusion criteria were the definitive diagnosis of PD with the clinical examination and clinical history of the patients as determined by a neurologist. The exclusion criteria included Parkinson's syndromes caused by neuroleptics or Parkinson's syndromes with known causes (vascular causes) that are not a primary idiopathic PD, as well as the patient's unwillingness to continue participating in the study.
3.3. Assessment of the Severity of PD
The severity of PD was assessed using the Unified Parkinson's Disease Rating Scale (UPDRS) and the Hoehn and Yahr Scale (HYS) according to Table 3 (18).
| Steps | Explanatory Steps |
|---|---|
| 1 | Assessment of thinking, behavior, mood |
| 2 | ADLs includes speech, food swallowing, handwriting, dressing, personal hygiene, a history of falling, the amount of saliva, sleeping, walking, and chewing food. |
| 3 | The clinical evaluation |
| 4 | Complications and treatment problems |
| 5 | Hoehn and Yahr scale |
| 6 | Schwab and ADL criteria |
3.4. Statistical Analysis
Data were analyzed using the SPSS software version 23 (SPSS, IBM Company, USA). Qualitative and quantitative statistical tests were used by this software to analyze the variables. The quantitative continuous variables were expressed as mean ± SD, and the distribution of qualitative frequency was expressed as percentages. Independent t-test was used to compare the serum levels of lead and cadmium in groups. Independent t-test is used to determine a statistically significant difference between the means in two groups. A P < 0.05 was considered as significant.
4. Results
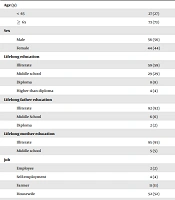
This case-control study was performed on 100 PD patients and 30 healthy controls. The demographic data of patients and controls are presented in Table 4. The mean age of patients and controls was 70.2 ± 9.8 and 71.5 ± 8.2 years, respectively. The duration of the disease in patients was 6.34 ± 4.61 years. Table 5 reports the severity and priority symptoms of the disease in different categories. The mean cadmium levels in the patients and the control groups were 14.91 ± 8.72 ppb and 4.71 ± 2.72 ppb, respectively (P < 0.001). The mean lead levels in the patients and the control groups were 158.35 ± 157.64 ppb and 35.35 ± 16.25ppb, respectively (P < 0.001) (Table 6).
| Group Variable | n = 100 (Patients) | n = 30 (Control) | P-Value |
|---|---|---|---|
| Age (y) | 0.440 | ||
| < 65 | 27 (27) | 6 (20) | |
| ≥ 65 | 73 (73) | 24 (80) | |
| Sex | 0.949 | ||
| Male | 56 (56) | 17 (56.7) | |
| Female | 44 (44) | 13 (43.3) | |
| Lifelong education | 0.615 | ||
| Illiterate | 59 (59) | 20 (66.7) | |
| Middle school | 29 (29) | 7 (23.3) | |
| Diploma | 8 (8) | 3 (10) | |
| Higher than diploma | 4 (4) | - | |
| Lifelong father education | 0.278 | ||
| Illiterate | 92 (92) | 30 (100) | |
| Middle school | 6 (6) | - | |
| Diploma | 2 (2) | - | |
| Lifelong mother education | 0.212 | ||
| Illiterate | 95 (95) | 30 (100) | |
| Middle school | 5 (5) | - | |
| Job | 0.433 | ||
| Employee | 2 (2) | - | |
| Self-employment | 4 (4) | - | |
| Farmer | 11 (11) | 3 (10) | |
| Housewife | 52 (52) | 13 (43.3) | |
| Unemployed | 31 (31) | 14 (46.7) | |
| Marital status | 0.181 | ||
| Single | 19 (19) | 10 (33.3) | |
| Married | 78 (78) | 20 (66.7) | |
| Death of consort | 3 (3) | - | |
| Residence | 0.168 | ||
| Urban | 36 (36) | 15 (50) | |
| Rural | 64 (64) | 15 (50) | |
| Smoke | 0.002 | ||
| No | 87 (87) | 25 (83.3) | |
| Yes | 2 (2) | 5 (16.7) | |
| Quit | 11 (11) | - |
a Values are expressed as No. (%).
| Variables | No. (%) |
|---|---|
| Severity of disease | |
| 1 -2 | 41 (41) |
| 2.5 - 3 | 43 (43) |
| > 3 | 16 (16) |
| Priority sign of the disease | |
| Tremor | 95 (95) |
| Bradykinesia | 90 (90) |
| Rigidity | 87 (87) |
| Gait disorder | 58 (58) |
| Variables | Patients | Controls | P-Value |
|---|---|---|---|
| Age (y) | 70.25 ± 9.83 | 71.50 ± 8.254 | 0.528 |
| Duration of the disease | 6.34 ± 4.61 | - | - |
| Cadmium level (ppb) | 14.91 ± 8.72 | 4.71 ± 2.72 | < 0.001 |
| Lead level (ppb) | 158.35 ± 157.64 | 35.35 ± 16.25 | < 0.001 |
a Values are expressed as mean ± SD.
According to Tables 7 and 8, there were significant differences in the lead and cadmium levels between the patients and healthy subjects in the age groups of under 65 and over 65 years. This was also observed in both genders, as well as urban and rural residences. Based on the priority signs of the disease, no significant difference was found between the patients with or without the disease signs. Also, there was no significant difference in the cadmium levels in different severities of the disease.
| Variables | Patients | Controls | P-Value |
|---|---|---|---|
| Age (y) | |||
| < 65 | 14.06 ± 7.78 | 3.60 ± 1.56 | 0.003 |
| ≥ 65 | 15.21 ± 9.07 | 4.99 ± 2.89 | < 0.001 |
| Sex | |||
| Male | 15.58 ± 8.35 | 4.11 ± 1.14 | < 0.001 |
| Female | 14.04 ± 9.18 | 5.50 ± 3.86 | 0.002 |
| Residence | |||
| City | 15.44 ± 9.68 | 5.09 ± 3.64 | < 0.001 |
| Village | 14.59 ± 8.19 | 4.33 ± 1.30 | < 0.001 |
| Tremor | 0.561 | ||
| Yes | 15.02 ± 8.84 | - | |
| No | 12.68 ± 6.17 | - | |
| Brydikinesia | 0.567 | ||
| Yes | 14.73 ± 8.80 | - | |
| No | 16.41 ± 8.18 | - | |
| Rigidity | 0.657 | ||
| Yes | 14.78 ± 8.18 | - | |
| No | 15.73 ± 8.10 | - | |
| Gait disorder | 0.281 | ||
| Yes | 15.70 ± 8.66 | - | |
| No | 13.79 ± 8.78 | - | |
| Severity of disease | 0.368 | ||
| 1 -2 | 15.49 ± 8.78 | - | |
| 2.5 - 3 | 13.69 ± 9.40 | - | |
| > 3 | 16.65 ± 6.43 | - |
a Values are expressed as mean ± SD.
| Variables | Patients | Control | P-Value |
|---|---|---|---|
| Age (y) | |||
| < 65 | 189.72 ± 207.07 | 29.36 ± 13.91 | 0.071 |
| ≥ 65 | 146.26 ± 134.79 | 36.85 ± 16.70 | < 0.001 |
| Sex | |||
| Male | 152.39 ± 134.36 | 33.57 ± 13.09 | 0.001 |
| Female | 165.133 ± 184.47 | 37.68 ± 19.97 | 0.017 |
| Residence | |||
| City | 163.64 ± 166.64 | 36.47 ± 16.13 | 0.005 |
| Village | 154.82 ± 153.73 | 34.23 ± 16.85 | 0.003 |
| Tremor | 0.455 | ||
| Yes | 155.27 ± 157.12 | - | |
| No | 209.69 ± 177.29 | - | |
| Bradykinesia | 0.090 | ||
| Yes | 149.07 ± 151.97 | - | |
| No | 238.32 ± 192.37 | - | |
| Rigidity | 0.715 | ||
| Yes | 160.72 ± 162.99 | - | |
| No | 139.78 ± 119.26 | - | |
| Gait disorder | 0.917 | ||
| Yes | 156.59 ± 146.42 | - | |
| No | 159.94 ± 173.76 | - | |
| Severity of disease | 0.960 | ||
| 1 - 2 | 155.20 ± 153.88 | - | |
| 2.5 - 3 | 153.55 ± 150.89 | - | |
| > 3 | 177.10 ± 191.43 | - |
a Values are expressed as mean ± SD.
5. Discussion
Based on the findings of this study, the lead and cadmium levels were significantly higher in PD patients than in healthy subjects (P < 0.001). Accordingly, there was no significant relationship between the severity of PD stages in the serum levels of the lead or cadmium, as well as the serum levels of the lead and cadmium in patients with the symptoms of tremor bradykinesia, rigidity, and gait disorder. However, those who did not have these symptoms were not significantly different.
In previous studies, exposure to lead has been accompanied by reduced intelligence, memory, analysis, reading, visual, motor, and other skills. Moreover, the exposure time of this metal was found to be effective in creating anxiety, depression, and phobias (11). Cadmium also plays a major role in neurobiology and poisoning, and it can have a potential role in neurodegenerative diseases, including PD. The neurotoxic role of the cadmium has been multiplied by the biochemical changes in the cells and functional changes in the CNS, which indicates the potential role of neurotoxic effects of systemic poisoning in exposure to chronic conditions (19).
In our study, the serum levels of lead and cadmium in PD patients were significantly higher than the control group. A significant difference in lead and cadmium levels between the patients and healthy subjects were observed in the age and gender categories. In urban patients, serum lead and cadmium levels were higher than in rural patients, but this finding was not significant.
There are some reports consistent with the present study (12, 20). Kumudini et al. measured the serum levels of several metals, including lead, in PD patients and healthy subjects (20). The serum lead levels in this study were twice as high as the normal, but no significant difference was found. They concluded that oxidative stress from metals such as lead can be a potential risk factor for PD. Also, consistent with our findings, they reported higher lead levels in urban patients and concluded that this was related to the industrial pollution and cars.
In another study (12), the risk of PD was twice as high in subjects exposed to high levels of the lead after adjusting for age, gender, race, smoking, coffee, and alcohol. The researchers analyzed the serum levels of lead and its bone concentration in PD patients, but no significant difference was found between the patients and healthy subjects. They reported that occupational exposure to the lead is strongly associated with the risk of PD, and finally concluded that lead can be a risk factor for PD.
There are several other studies investigating the effects of lead or cadmium in diseases such as PD (19, 21-28). In 2014, Palacios et al. examined a group of nurses who had exposure to neurotoxins, including lead and cadmium, from 1990 to 2008 to assess PD risk. No significant association was found between the exposure to these metals and an increased PD risk (23).
In a study conducted by Willis et al., the PD occurrence was higher in the states with higher levels of pollution, while in states with a low level of pollution, the incidence of disease was lower. They reported that they were not able to establish a direct link between the PD and lead industrial exposure, but suggested that the lead is still a neurotoxin for the basal ganglia and is a risk factor for PD (22).
Dhillon et al. investigated the possible association of PD in subjects exposed to pesticides and cadmium, and concluded that exposure to cadmium in pesticides correlated with the risk of PD (21). Gorell et al. determined the association between occupational exposure to multiple metals and the PD risk. Their results showed that chronic contact with metals such as manganese and the copper alone or in combination with the lead was associated with an increased risk of PD. Also, the lead alone was not a risk factor for PD (25-27). In an overview by Bjorklund et al. in 2018, the role of metals such as mercury and lead in the occupational exposure and people suffering from PD was discussed. It was also mentioned that the combination of metals such as mercury with lead and cadmium can increase production of various oxidative factors (28). In a study by Firestone et al., there was no relationship between exposure to the lead alone or in combination with metals such as copper and manganese with the risk of PD (24).
While some studies indicated that exposure to lead or cadmium is not associated with the risk of PD, several other studies concluded that lead in combination with other metals can increase the risk of PD.
In our study, the number of healthy subjects was relatively low (n = 30) compared to the patients (n = 100). Also, we were not able to evaluate the exact source of the metals exposure in included subjects.
5.1. Conclusion
Based on the findings of this study, there was a higher level of serum lead and cadmium in PD patients compared to the control group. This finding was observed for both genders and at different ages. Therefore, it seems that exposure to lead and cadmium may be associated with an increased risk of PD. It is extremely recommended that groups exposed to the lead and cadmium, including occupational exposures, be considered for PD risk.