1. Background
Multiple sclerosis (MS) is a disabling and chronic disease associated with inflammation and demyelination, and neurodegeneration of the central nervous system (CNS) in humans (1). The onset of the disease is accompanied by inflammation and infiltration of immune cells into the CNS and damage to the blood-brain barrier. In the long term, multiple damages to oligodendrocytes, axons of the spinal cord, and brain neurons lead to an irreversible lesion, and plaques appear in the CNS (2). The white matter lesions are formed around the inflamed veins confirmed by high field MRI (3, 4). Actually, MS is primarily a white matter disease, but the significant neuronal loss has also been demonstrated in grey matter MS lesions. Accordingly, cortical lesions with perivenous inflammation are found in early and advanced MS cases (5). Some types of cortical lesions are also described in the forebrain/cerebellum, including intracortical, leukocortical, and subpial lesions (6-9). Active demyelination is also characterized in meningeal membranes by producing inflammatory infiltrates to diffuse into the cortex and trigger demyelination through microglia activation (10). Similar cortical and neocortical lesions associated with meningeal inflammation have been essentially induced in specific rat EAE models (11, 12). The MRI studies also recommend a major clinical relevance of cortical pathology in the forebrain of the MS patients, and it seems that widespread demyelination in the cerebellar cortex is involved in those with the primary or secondary progressive of disease (13). Actually, different patterns of brain injury have been shown in patients with MS, and some of them are associated with the production of reactive oxygen/nitrogen species in inflammatory CNS demyelination (14). The virtual hypoxia and chronic necrosis of demyelinated axons in MS lesions are related to energy failure due to the oxidative damage and mitochondrial dysfunction (15). Ultra-structural analysis of demyelinated spinal cord lesions revealed a significant reduction in the number of mitochondria and microtubules and axonal swelling (16). Proteome analysis of the brain of the EAE animal models also indicated significant defects in mitochondrial respiratory chain complex function, especially they are detectable in some subunits of the COX enzyme (17). Davies et al. tried to explore the presence of actual tissue hypoxia in the rat model of EAE. They claimed that the neurological deficit in CNS of the EAE is closely correlated with hypoxia. Transcription factors such as hypoxia-inducible factors (HIFs) play a central role in regulating and coordinating the cellular response to hypoxia (18). HIF activity is thought to be controlled by the activity of its α-subunit, which is essentially regulated in an oxygen-dependent manner (19). The stability and activity of HIF-1α are regulated by various post-translational modifications (20). Under normoxic conditions, HIF-1a is hydroxylated by oxygen-dependent proline hydroxylases and rapidly destroyed (21), but under hypoxia condition, HIF1A expression is often significantly up-regulated through a redox-sensitive mechanism and forms a more stable active dimeric transcriptional complex. Therefore, HIF-1α is recognized as a marker for hypoxic tissue damage (22). Several enzymes responsible for metabolic changes towards anaerobic glycolysis are directly controlled by HIF-1α to reduce mitochondrial oxygen consumption (23). The HIF-1α also regulates mitochondrial respiration by changing the composition of COX in hypoxic conditions (24). In support of this matter, an enhanced number and activity of mitochondria have been reported in MS lesions (25).
2. Objectives
Since the provision of oxygen and energy to the brain by mitochondria of neurons is of crucial importance, investigating the consequences of mitochondrial dysfunction in the brains of the EAE mice model of MS would be interesting. Considering the importance of the COX in the electron transport chain of the brain cells, as well as the role of inducing HIF-1α in hypoxia–like conditions, we hypothesized the probable mitochondrial dysfunction in whole-brain cells of EAE mice model of MS disease, with decreasing COX and ATP levels could increase HIF-1α level, as well.
3. Methods
3.1. Animals
All experiments on the animal groups were approved by the regional ethics committee for animal research of the faculty of Medical Sciences of Tarbiat Modares University, Tehran (Iran). Twenty-one C57BL/6 female mice with 7 weeks of age were prepared from the Pasteur Institute of Iran. Animals were kept in a specific pathogen-free unit with 12/12 hours of dark/light cycles at a temperature of 20 to 24°C with adequate water and standard foods and were allowed to acclimate for five weeks in the animal house before any injections. Mice were divided into three experimental groups as follows: nine for the EAE model, six for the negative control, and six for the sham control groups.
3.2. EAE Induction Model
Nine mice (12 weeks old) in the EAE group were immunized with Hooke kitTM (EK-2110, MOG35-55/CFA emulsion, Pertussis toxin (PTX), Hooke laboratories, MA, USA) according to the manufacturer's protocol. Mice were first anesthetized with isoflurane, and then specific emulsion containing Myelin Oligodendrocyte Glycoprotein35–55/complete Freund's adjuvant (MOG35–55/CFA) was subcutaneously injected into both flanks of each mouse (0.2 ml/animal). Thereafter, each mouse was injected by intraperitoneal (IP) manner with pertussis toxin on the same day, after 2 h and 24 h later (400 ng in 0.1 ml/mouse/injection).
3.3. Control Group
Six mice (12 weeks old), as the negative control group, were anesthetized, and 0.1 ml of CFA emulsion (without MOG35–55 peptide) was subcutaneously injected into their both flanks (0.2 ml/mice) as the same manner performed for the EAE group. Thereafter, the PTX was also injected by the IP manner as described for the EAE group.
3.4. Sham Group
Similar to the control group, six mice were anesthetized, and only 0.1 ml of PBS was subcutaneously injected into their both flanks (0.2 ml/mouse), as well. Then, each mouse received IP injections of PBS buffer on the same day, after 2 and 24 h later (0.1 ml/mouse/injection).
3.5. Clinical Score Evaluation
Typically, the EAE induction group is scored on a scale ranging from 0 to 5, as indicated in the Hooke kitTM. Hence, all animal groups in this study were scored for the disability MS disease from day 5 to day 14 (the sacrificing day) of immunization, while were checked by two researchers who were blinded to the administered treatments using the 10-point EAE scoring system, which is as follows. Zero, no signs of the clinical disease; 0.5, partial tail paralysis; 1.0, complete tail paralysis; 1.5, complete tail paralysis and discrete hind limb weakness; 2.0, the same paralysis with strong hind limb weakness; 2.5, unilateral hind limb paralysis; 3, complete hind limb paralysis; 3.5, hind limb paralysis and forelimb weakness; 4.0, complete paralysis; and finally 5.0: moribund or dead (26).
3.6. Isolation of the Brain Tissue
All brain tissues of 3 groups of mice were separated, divided, washed by PBS, and then immediately entered into the nitrogen tanks and transferred to -80°C.
3.7. Measuring the Total Protein Content of the Centrifuged Mice Brain Tissues
The extraction of total native proteins from all mice brains was performed by the 3-min Total Protein Extraction Kit (Cat No: P502L, 101 Bio, USA) according to the manufacturer instructions. Briefly, 15 - 20 mg of the frozen mice brains of each group was incubated for 5 min on ice after being crushed and homogenized by the pestle device. Then, all samples were centrifuged at 10,000 g for 3 min at 4°C. The total proteins of pellets and supernatants were measured by the Bradford method.
3.8. Specific COX Activity in Supernatants / Pellets of the Centrifuged Mice Brain Tissues
Total COX activity in the centrifuged mice brains of each group was first measured spectrophotometrically using the COX assay kit (Sigma-Aldrich, USA) according to the manufacturer's instruction. Briefly, 20 to 40 μl of those samples were mixed by inversion with the 950 μl of assay buffer and 60 - 80 μl of the enzyme dilution buffer. Then, 50 μl of ferrocytochrome C substrate solution was added to start the reaction at room temperature (RT), and immediately the absorbance changes were recorded for 1 min at 550 nm using a kinetic program. One unit of the COX could oxidize 1.0 μM of ferrocytochrome C per min at pH 7.0 at 25°C. Since the outer membrane of mitochondria is a barrier for the entrance of cytochrome C into the organelle, the n-dodecyl beta D-Maltoside detergent, which allows increasing maintenance efficiency of the COX multimer structure, was used for enzyme assay in pellets of the extracts.
3.9. ATP Measurement
This assay was performed for all mice brains using the ATP colorimetric/fluorametric assay kit (Sigma-Aldrich, USA), according to the manufacturer's instructions. Briefly, an appropriate frozen mice brain lysed in the ATP assay buffer, provided in the kit, immediately after being homogenized and thawed. The deproteinized suspensions were centrifuged at 13000 g for 2 min at 4°C. Afterward, 44 µl of collected supernatants added to 96 well plates. Then, the ATP probe, ATP converter, and developer mix were added up to 50 µl to each well, and were mixed and incubated at RT for 30 minutes. The optic density of each sample was measured at 570 nm in the plate reader. Finally, the ATP content of each sample was calculated with respect to an ATP standard curve.
3.10. HIF-1α Measurement
Specialized ELISA kit (Mouse HIF-1α, ZellBio GmbH, Germany) with sensitivity up to 5 ng/L and assay range of 150 to 4800 ng/l was used to measure the HIF-1α level in mice brains, according to the manufacturer's instruction. Briefly, some other frozen brains were immediately homogenized in PBS and centrifuged at 5000 g for 10 min. Supernatants were conducted immediately for measuring the content of the HIF1-α in 3 experiment groups with respect to the standard curve of original HIF1-a provided in the kit.
3.11. Statistical Analysis
Data were analyzed by SPSS V19 software. The normal distribution of data was assessed using the Kolmogorov-Smirnov test, and then, data differences between the mean of each group of mice brains (Sham, control, and EAE) were analyzed by the paired-sample t-test. Also, P values less than 0.05 were considered as statistically significant.
4. Results
4.1. Clinical Scores
The early-onset of disease was observed on day 8 of immunization in six (out of seven) mice of the EAE group. The clinical score of each EAE mouse increased gradually until day 14; the score of five mice reached to 3 - 3.5 on the 14th day of the immunization. The overall clinical score variations in the EAE mice group over a 14-day time period was in accordance with the data provided by the EAE induction kit supplier (Hooke EAE induction kit). Meanwhile, mice in the sham and control groups did not show the onset of disease, and their scores remained at zero throughout the experiments.
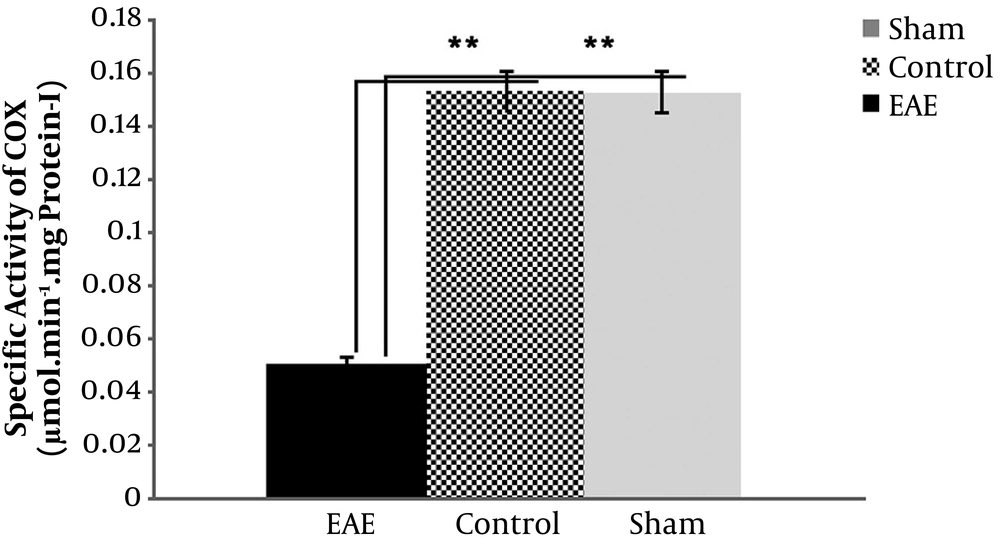
4.2. Specific COX Activity in the Homogenized Mice Brain Tissues
Data obtained from the pilot study on the measurement of specific COX activity in the centrifuged mice brain extracts showed that the pellets would be the best, up to 5 times more than supernatants, so the project focused on the pellets for this purpose. Data indicated a significant decrease in the specific activity of COX in the pellets of the EAE model (P value < 0.001), more than 3 times compared to the sham and negative control groups (Figure 1). The data also showed no significant difference between sham and control variables (P value > 0.05).
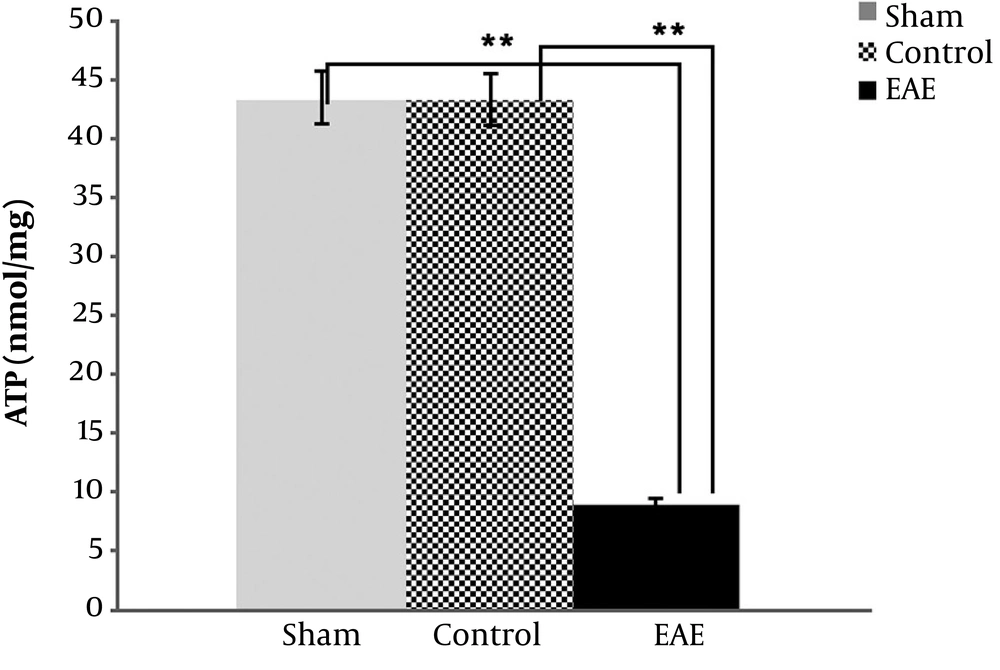
4.3. ATP Measurements in Three Mice Groups
Data also reconfirmed the above findings and indicated that ATP values in the EAE samples were highly significantly reduced compared to the negative and sham controls (P value < 0.001), as expected (Figure 2). Meanwhile, no significant difference was found between sham and negative control variables (P value > 0.05).
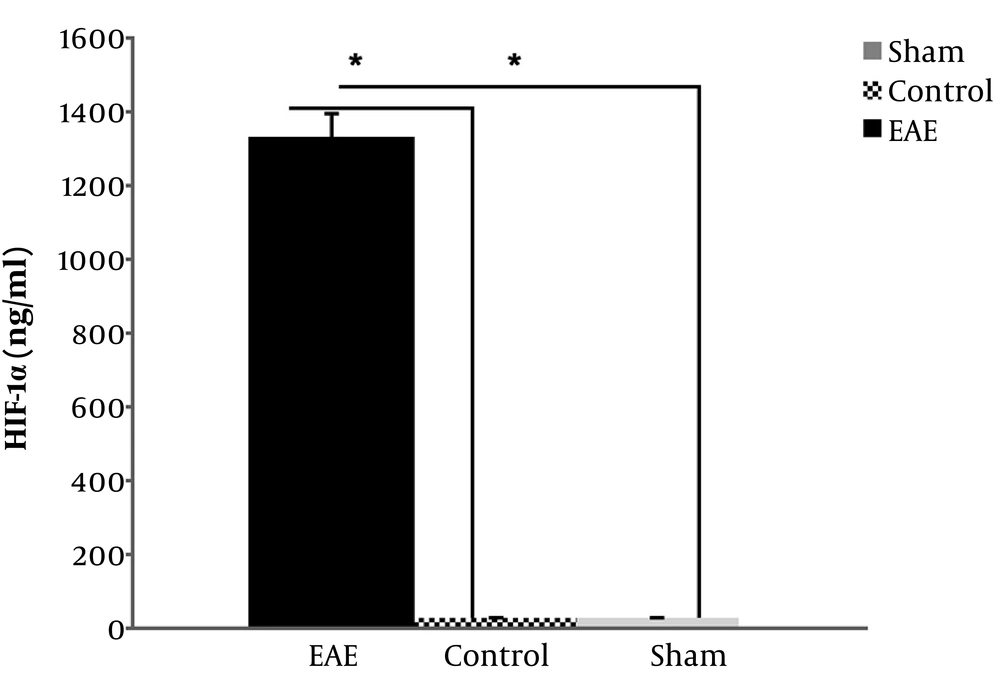
4.4. HIF-1α Measurements in Three Mice Groups
In the case of values of HIF-1α in examined EAE and controls groups, the data indicated a significant increase of this factor in the EAE group compared to both negative control and sham groups (P value = 0.037, P value = 0.031, respectively), which implies the presence of hypoxia-like conditions in EAE mice brains, as well (Figure 3). However, there was no significant difference between the sham and negative controls (P value = 0.250).
5. Discussion
Several studies have shown that degenerative mechanisms may be due to mitochondrial dysfunction (27). Related phenomena, such as radical changes, have been elucidated in MS pathogenesis (28), and reduction of COX gene expression in the blood of MS patients was observed (29). One of the important results of the present study is the significant reduction of ATP value in the EAE group, compared to the negative and sham controls. The lowering ATP can be attributed to the low specific activity of COX, which is determined and confirmed. In 2010, proteome analysis of the EAE mice brain revealed a significant reduction of expression of two subunits of the COX enzyme (Cox5b, Cox5a) involved in the structure and accumulation of complex IV of mitochondria (17). The authors concluded that low energy in EAE mice's brains is similar to the hypoxia-like damage that occurred in the CNS of the MS patients. These conditions could be characterized by a gradual increase of the HIF-1α and accumulate in hypoxic conditions in associating with HIF-1β to form a functional complex, triggering to do transcription a large collection of hypoxia-inducible genes involved in nerve protection leading to better adaption, and survival of cells exposed to hypoxia. HIF-1α may increase blood oxygen, glucose supply, compensate for the reduced ATP supply with glycolytic pathways by regulating their genes (30). In the case of animal models, mice deficient for HIF-1 showed significantly less neuronal cell loss than control mice in response to hypoxia (31). Plenty of evidence indicated that potential neuroprotective roles of HIF-1α are involved in the pathogenesis of Alzheimer’s, stroke, and inflammatory brain diseases (32-35). In spite of the several reports about the activation/inactivation of HIF-1α in cell-mediated inflammation, the specific role of HIF-1α in the pathogenesis of the disease is still unknown (36). However, autopsy and microarray studies on the MS patients indicated that mitochondrial damage and related hypoxia-like conditions in white matter are the important pathway of tissue injury in MS disease. Oxidative damage, ROS, and NO has been implicated and used in the explanation of hypoxia-like observations (37, 38). One of the important reports is the actual hypoxia in the white matter of the CNS of the EAE rat model presented by Davies in 2013. They tried to assess the brain hypoxia in vivo and reducing the hypoxia-like condition by administration of brief and continues normobaric oxygen (18). They also tried to administrate the iNOS inhibitors by borrowing the method of Zielasek et al., to improve the hypoxia-like condition, as well (39). They concluded that the neurological deficit was closely correlated with spinal white and gray matter hypoxia. Consistent with this finding, we planned to assess the HIF-1α, ATP, and specific activity of COX enzyme in whole-brains of the EAE mice model of MS. The data also showed that HIF-1α was significantly increased in the EAE mice group, compared to the negative control and sham groups. The only report that is not relatively consistent with these data is the published paper by Moan (2015) (40), who described in spite of the significant increase of the HIF-1α in astrocytes and myeloid in spinal cord samples of the EAE mice, the expression level of this factor in those cells was not necessary for the development of the neuro-inflammatory disease. However, there are important reports which showed that induction of HIF-1α may be influential, as the neuroprotective agent for other neurodegenerative disorders such as Alzheimer's disease (32) or as a therapeutic target in the ischemic stroke (34). In 2014, Li et al. described that up-regulated HIF-1α may be involved in the process of epileptogenesis but not in the acute stage of epilepsy in animal models and claimed that the modulation of HIF-1a may offer a novel therapeutic target in epilepsy (41). In 2018, Navarrete et al. tried to use a special compound (VCE-004.8) to mimic hypoxic conditions by stabilizing the HIF-1α and HIF-2α as well as activating the HIF pathway in different cell types of two animal models of MS. In vivo experiments of this study revealed that the VCE-004.8 treatments could prevent demyelination, axonal damage, and immune cell infiltration (42). It seems the HIF-1α induction may also be a potential target to control MS progression.
In conclusions, the present study, for the first time, showed that in peak score of MS disease in the EAE mouse model, specific COX activity and, consequently, ATP levels of the mice brain cells were significantly decreased, indicating the presence of hypoxia-like conditions. Significant induction of HIF-1α in the EAE mice brains also implies indirectly hypoxia-like conditions. This indicates that enhanced HIF-1α may compensate for reduced ATP supply resulted from the COX activity loss in compromised mitochondria and could prevent neuronal death under hypoxia-like conditions in the EAE mice brains. In general, it seems that these data may help to elaborate on the role of HIF-1α as the neuroprotective agent for MS disease.