1. Background
Parkinson is the second most frequent neurodegenerative (after Alzheimer’s disease). Its incidence is less than 1% among those aged 60 or more. Most patients have motor disorders, particularly bradykinesia, tremors during rest, rigidity, and disorders of posture and the gait (1).
The prevalence of Parkinson appears to be higher in Europe, and North and South America. Its prevalence is estimated to be about 66-1500 cases per 100 000 population (2), 111 - 329 cases per 100 000 population (3), and 31 - 470 cases per 100 000 population, respectively (4), respectively; as compared to African, Asian, and Arabic countries, where the prevalence of Parkinson is in the range of 10 - 43 cases per 100 000 population (5), 15 - 119 cases per 100 000 population (6), and 27 - 43 cases per 100 000 population (7), respectively. Also, its incidence ranges from 10 to 18 cases per 100,000 person-years (8).
Parkinson is most certainly multifactorial in nature, but complex interactions between various factors are not identified yet. The most important risk factor for the development of Parkinson’s is old age, so that it can be said that the prevalence and incidence of the disease increase by age, with a peak at the age of 80 years (9, 10). In addition, gender (11) and ethnicity (8) are also reported as risk factors for the development of Parkinson’s disease.
The prevalence and the clinical profile of Parkinson’s disease in Morocco are still far from been established. However, in 2016, about 20839 individuals were diagnosed with Parkinson’s disease in the country. A 37.9% increase since 1990 (12), and its prevalence is expected to increase further, in correlation with the aging of the population. In particular, those who are living in the southern part of the country are shown to have a specific demographic and genetic characteristic, distinct from other regions in Morocco.
2. Objectives
To improve or support the existing data, the current cross-sectional study which was conducted in the south of Morocco, aimed to investigate the clinical profile and to determine if male patients with Parkinson’s disease have a different presentation.
3. Methods
3.1. Patients Selection
This is a cross-sectional study on a cohort of 180 patients, previously diagnosed with Parkinson’s disease. The patients were recruited from both private and public neurology centers in the city of Agadir, south-west of Morocco. Written consent was taken from all participants, according to the recommendation of the Ethics Committees of the Faculty of Medicine and Pharmacy of Marrakech.
3.2. Data Collection
Data were collected from January 2017 to July 2018, using a detailed clinical questionnaire which was filled up by clinicians through interviews with the participants. The severity of the disease was assessed using the Hoehn and Yahr scale. The cohort was sampled into five study groups, according to their age (40 - 50 years old, 51 - 60 years old, 61 - 70 years, 71 - 80 years, and ≥ 80 years) and to the age of onset of the disease (30 - 40 years, 41 - 50 years, 51 - 60 years old, 61 - 70 years old, and 71 - 80 years). The questionnaire was previously tested in a small sample of patients before the data collection. To ensure the quality of the collected data. Regular contact between the investigators and the neurologist was maintained throughout the study.
3.3. Statistical Analysis
Data were analyzed using various software and statistical tests. The normality of data was tested using the Kolmogorov Smirnov test. The demographic and clinical differences between both genders (Male and Female) were assessed using Welch’s two-sample t-test test for continuous data. The continuous variables, if not following a normal distribution, were presented as a median (quartile) and compared with a non-parametric test. The differences between categorical distributions were assessed using the chi-square test or the Fisher exact test. Spearman’s correlation was used to quantify the strength of the linear association between continuous variables. A P value lower than 0.05 was considered as statistically significant. The statistical analyses were conducted using the IBM SPSS statistics software 22.
4. Results
4.1. Repartition of the Patients According to Gender
In total 180 patients participated in the current study (117 males and 63 females). Male patients were twice the females. Accordingly, the frequency of male patients was higher compared to females (65% and 35 %, respectively). Sex ratio was estimated to be around a mean of 1.85:1, in favor of the males. However, the sex ratio varied according to the age of the patients, increasing from 0.71:1 in favor of females in the age range of 30 to 40 to 2.52:1 in favor of males in the age range of 61 to 70 years old. For a detailed analysis, see Table 1.
| Variable | Overall (N = 180) | Male (N = 117) | Female (N = 63) | P Value |
|---|---|---|---|---|
| Mean age in years (41 to 92 years) | 68.1 (± 11.3) | 68.9 (± 10.4) | 66.6 (± 12.8) | 0.21a |
| Age (years) | 0.02b | |||
| 40 - 50 | 12 (6.7%) | 5 (4.3%) | 7 (11.1%) | |
| 51 - 60 | 31 (17.2%) | 18 (15.4%) | 13 (20.6%) | |
| 61 - 70 | 59 (32.8%) | 42 (35.9%) | 17 (27%) | |
| 71 - 80 | 52 (28.9%) | 36 (30.8%) | 16 (25.4%) | |
| > 80 years | 26 (14.4%) | 16 (13.7%) | 10 (15.9%) | |
| Mean age of onset of the disease | 60.3 (± 9.7) | 61.4 (± 9.3) | 58.3 (± 10.4) | 0.04a |
| Duration of the disease | 7.92 (± 5.67) | 7.57 (± 5.22) | 8.56 (± 6.42) | 0.29a |
| Family history | ||||
| Familial PD | 43 (24%) | 28 (23.9%) | 15 (23.8%) | 0.5b |
| Family tremor | 22 (12.2%) | 15 (12.8%) | 7 (11.1%) | - |
aStudent test
bFisher test
4.2. Age at the Onset of the Disease in Correlation with the Gender
The results showed that the age at the onset of the disease ranges from 35 to 80 years old. The mean age at the onset of symptoms for males and females was 60.3 ± 9.78. However, the age of the onset of Parkinson’s disease was found to be higher in male patients (61.4 ± 9.28), compared to females (58.3 ± 10.4) (P < 0.05, t = 2.012). According to the collected data, the mean of the duration of the disease nearly (7.92 ± 5.67) years, with a range of 0.5 - 30 years. The P value (0.29) of difference between males and females was not significant (P > 0.05, t = -1.119). For a detailed analysis, see Table 1.
4.3. The Correlation Between the Gender and the Symptoms at the Onset of the Disease
The most important clinical motor signs of Parkinson’s disease include tremor, rigidity, and bradykinesia. In this study, the most common initial symptoms were tremor (50%, n = 91), rigidity (33%, n = 59), and bradykinesia (17%, n = 30), respectively (Table 2). However, 89 patients did not show any sign of tremor at the onset of the symptoms. The comparison between males and females concerning the initial symptoms showed that the female population was the most represented, the tremor symptoms at the onset of disease; tremor (58.7%, n = 37/63 P = 0.04). In contrast, the prevalence of bradykinesia was fewer among females (15 (23.8%)) at the beginning of the disease, compared to males. The calculated P value suggests a correlation between the tremor and bradykinesia symptoms and gender.
| Variables | Initial Symptom | |||||
|---|---|---|---|---|---|---|
| Tremor | P Value | Rigidity | P Value | Bradykinesia | P Value | |
| Gender | 0.04a | 0.04a | ||||
| Male | 54 (46.1%) | 19 (16.2%) | 0.4a | 44 (37.6%) | ||
| Female | 37 (58.7%) | 11 (17.4%) | 15 (23.8%) | |||
| Age at onset of disease | 0.3a | 0.2a | ||||
| 30 - 40 | 3 (3.3%) | 1 (3.3%) | 2 (3.4%) | |||
| 41 - 50 | 12 (13.2%) | 5 (16.7%) | 8 (13.6%) | |||
| 51 - 60 | 33 (36.3%) | 7 (23.3%) | 0.5a | 22 (37.3%) | ||
| 61 - 70 | 23 (25.3%) | 13 (43.3%) | 20 (33.9%) | |||
| 71 - 80 | 20 (22%) | 4 (13.3%) | 7 (11.9%) | |||
aFisher test
4.4. The Correlation Between the Age of the Patients and Their Initial Symptoms, Clinical form and the Hoehn and Yahr Stages of the Disease at the Onset
We investigated both the initial symptoms, clinical form, and the severity of the disease using the Hoehn and Yahr scale scoring at the onset of disease. The results (Table 2), indicated that all symptoms (tremor, rigidity and bradykinesia) were presented from 41 and 80 years old. However, these results showed that the rigidity symptoms were slightly higher from 61 and 70 (43.3%, n = 13/30) and 51 to 60 years (23.3%, n = 7/30); respectively. Simultaneously, the results showed that the tremor and bradykinesia were mostly associated with age intervals of 61 to 70 and 71 to 80 years old.
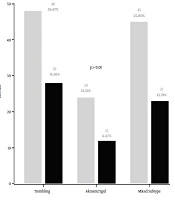
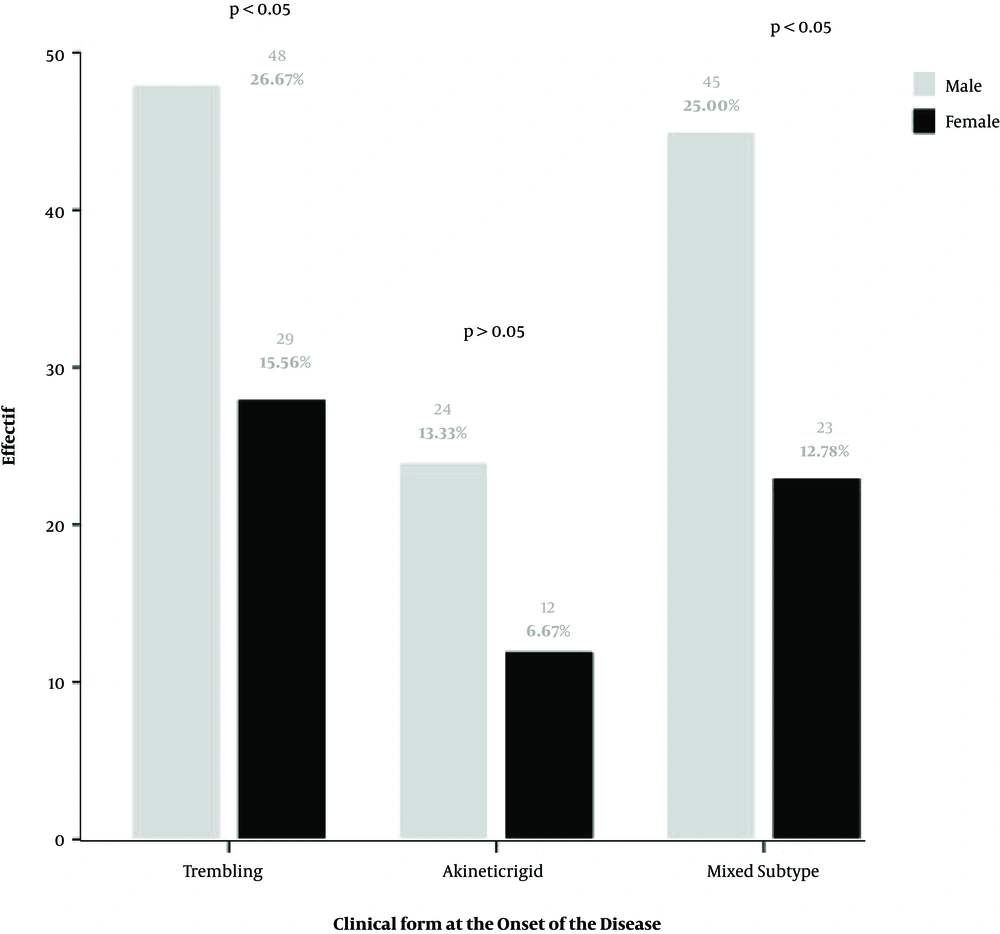
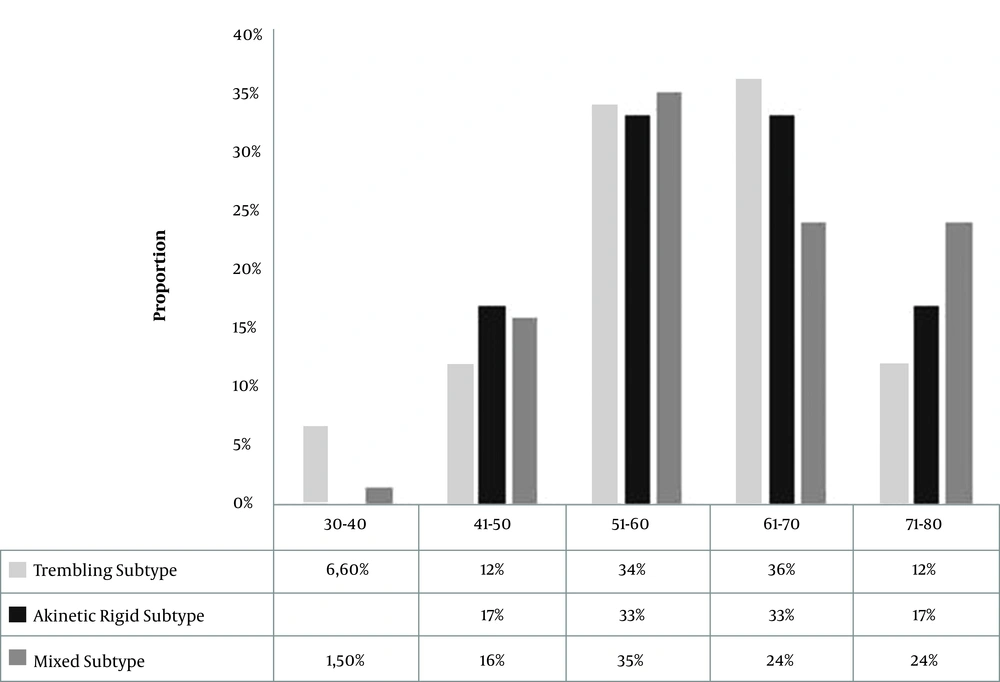
The distribution of the clinical form of the disease by gender is shown in Figure 1. According to the results, the trembling subtype was the most frequent symptom for both males and females (n = 76, 42%), compared to the mixed subtype (n = 68, 38%), and the akinetic-rigid subtype (n = 36, 20%), respectively. According to the age at the onset of disease, the akinetic rigid subtype was found in all age intervals, except for younger patients (i.e., 30 - 40 years old). At the same time, the mixed form was more prevalent among older patients (71 - 80 years old). Also, we found that three forms of the disease were equally represented at the age intervals between 41 and 70 years old of the onset of the Parkinson (Figure 2).
The severity of the disease was assessed using the Hoehn and Yahr scales, which allowed us to score the severity from mild (1-2), to moderate (2.5-3), and advanced (4-5). According to the results, the mean score of the total population was 1.77 ± 1,08. According to the Hoehn and Yahr scales, the majority (82.6%, n = 147) of the patients of both sexes were in the early stage of the disease (stages 1 and 2 of H & Y). While only 10% (n = 18) were in the advanced stages of the disease (H & Y 4-5). The calculated P value suggested that the severity of the disease was strongly dependent on gender (P = 0.02) (Table 3). The relative duration of the disease was estimated to be less than 5 years for 36% of the participants, between 5 to 9 years for 27%, and more than 9 years for 37%. The results showed that the severity of the disease increased in correlation with the duration. Indeed, we found that 83% of patients with the highest Hoehn-Yahr score had a longer duration of disease (9 years or more) (P = 0.01). For a detailed analysis see Table 4. Additional statistical analyses conducted using the Spearman’s correlation confirmed a strong positive correlation between the Hoehn Yahr stages of the disease and both duration (P < 0.001) and the age of the patients (P < 0.05). Additional data are listed in Table 5.
| Variable | Stage of disease Hoehn & Yahr Scale | P Value | ||||||
|---|---|---|---|---|---|---|---|---|
| 1 | 1.5 | 2 | 2.5 | 3 | 4 | 5 | ||
| Gender | 0.03a | |||||||
| Male, No. (%) | 39 (33.3) | 52 (44.4) | 4 (3.4) | - | 11 (9.4) | 8 (6.8) | 3 (2.5) | |
| Female, No. (%) | 24 (38.1) | 24 (38.1) | 4 (3.4) | 1 (1.5) | 3 (4.7) | - | 7 (11.1) | |
| Total, No. (%) | 63 (35) | 76 (42.2) | 8 (4.4) | 1 (0.5) | 14 (7.7) | 8 (4.4) | 10 (5.5) | |
aFisher test = 13.79
| Variable | Midle (N = 148) | Moderate (N = 14) | Advanced (N = 18) | N | P | Test |
|---|---|---|---|---|---|---|
| Gender | 0.09 | Fisher | ||||
| Male | 96 (64.9%) | 10 (71.4%) | 11 (61.1%) | 117 | ||
| Female | 52 (35.1%) | 4 (28.6%) | 7 (38.9%) | 63 | ||
| Duration of disease | ||||||
| < 5 years | 61 (41%) | 3 (21%) | 0 (0%) | 64 | < 0.001 | Fisher |
| [5 - 9] years | 41 (28%) | 5 (36%) | 3 (17%) | 49 | - | - |
| > 9 years | 46 (31%) | 6 (43%) | 15 (83%) | 67 | - | - |
aMidle stage (stage 1 - 2), moderate (stage 2.5 - 3), advanced (stage 4 and 5)
5. Discussion
In total 180 patients participated in the current study, which based on their gender were divided into two groups: males (n = 117) and females (n = 63), with a sex ratio of 1.85 in favor of males population. The mean age of the patients was 68.1 ±11.3 years. The mean age of the females was lower than that of males (66.6 and 68.9 years, respectively). At the same time, the mean age at the onset of the disease for the total population was nearly 60.3 years (± 9.7). Meanwhile, comparing the mean age at the onset of the disease showed that Parkinson’s disease started earlier in females than males (58.3 ± 10.4 and 61.4 ± 9.3 years, respectively). Interestingly, 17.4% of the total population contracted Parkinson's disease earlier than the age of 50. Also, the findings showed that only 23.3% of the patients had a family history of Parkinson. This suggested that non-genetic risk factors might also affect the predisposition to the disease in the population of the south of Morocco. We did not find any significant difference between male and female groups.
Consistent with previous reports (13), the sex ratio was highly in favor of the male group (1.85), suggesting a possible hormonal neuroprotection mediated by the secretion of oestrogen in women (14). In addition, males might be more exposed to environmental risk factors due to the nature of their professional occupation. We found that the mean age at onset of the disease, in the population of the south of Morocco, was higher compared to that reported in other studies in Morocco(15). This relative difference might be due to either the importance of the sample size of the current study or to access better health care infrastructures in the capital city of the country.
In agreement with other studies (15, 16), we showed that women contracted Parkinson’s three years earlier than males. However, the mean age at the onset of the disease was lower compared to that previously reported in Asia (17). We also found that females with Parkinson in the south of Morocco were younger compared to female patients in North African countries (18). This difference can be attributed to the genetic profile of the population, particularly since studies reported that the mutation (G2019S) of the LRRK2 gene is an important genetic determinant of Parkinson’s disease occurrence in the population living in the south of Morocco. This was supported by the positive family history of the participants in 23% of patients. Early-onset of the Parkinson was found in 17.7% of the participants in the cohort, which is consistent with previous reports, which reported that 25.5% of the cases with an earlier onset of Parkinson had a family history of the disease (19).
The onset of dyskinesia and motor fluctuations are major problems for the long-term health of patients with Parkinson’s disease. Different patients present various manifestations of Parkinson (20). In the present study, the most frequent motor symptoms experienced by the patients, in both gender, were: tremor (50.6%, n = 91), bradykinesia (32.8%, n = 59), and rigidity (16.6%, n=30). This indicates that the initial symptoms of male patients differ from that of females. Indeed, the comparison between males and females concerning the initial symptoms showed that males experienced more significant symptoms of tremor and bradykinesia compared to females. Besides, we showed that the prevalence of initial symptoms was different from that described in previous studies (15, 21, 22). In addition, according to the findings, the prevalence of rigidity symptoms was slightly higher in younger patients (41 - 50 years old) and patients aged 61 to 70, at the onset of the disease. At the same time, we showed that the tremor and bradykinesia were significantly more prevalent from 51 - 60 and 71 - 80.
Considering the clinical pattern of Parkinson’s disease in the South of Morocco, we found that both the trembling form and the mixed type form were the most common types, with a slight difference. By contrast, the akinetic-rigid form was the less common, in agreement with other studies (23, 24). However, it was also reported that the akinetic–rigid and tremor-dominant subtypes were more common than the mixed (21). The clinical forms of the disease were changing with age. In the patients younger than 40 years, we found only trembling and mixed form. At the same time, the mixed form was associated with the late onset of the disease. We also showed that the severity of the disease was strongly related to gender, and increased in correlation with increased duration of PD and the age of the patients. By contrast, in the ages of around 41 years, all three forms were prevalent.
Consistent with other reports, a large number of patients (82.6%) were in the early stage of the disease (25). According to the Hoehn & Yahr scale of severity, we found a significant positive correlation between the severity of the disease and the age of the patients. This confirms the findings of the previous that showed the influence of age on the Hoehn Yahr scale (26).
5.1. Conclusion
We concluded that several clinical manifestations could be associated with the patients with PD in the south of Morocco. The current study confirmed the hypothesis that there are differences in clinical characteristics between the two genders. Overall, males and females had differences in clinical motor characteristics in the initial symptom of Parkinson’s disease progression, age of onset, and severity of the disease. Future research should investigate whether gender affects the clinical profile in the progression of the disease. Nevertheless, experimental studies must be carried out to achieve a real understanding of what underlies these differences.