1. Background
Remifentanil is a μ-opioid receptor agonist, which is recognized as a cardinal drug widely using in the total intravenous anesthesia (TIVA) method (1). It also can enhance the quality of anesthesia in those without a cancer diagnosis. Opioids are commonly using for maintaining general anesthesia in the United States (2-4). However, widespread using of opioids and their non-medical misuse have raised concerns about the harms and drawbacks of opioids, including remifentanil (4). Opioid-induced hyperalgesia (OIH) is a major consequence that has attracted the attention of many researchers (5). OIH is a major complication of continuous or intermittent opioid administration, which in turn increases the sensitivity to pain. This condition paradoxically enhances the sensitivity to pain, which is alleviated by lowering opioid doses. The pain pattern may be identical to the pain due to the underlying disease or appears different in quality. The patient may feel the pain in a different location(s) or use neuropathic pain descriptors (6). Although many animal or clinical studies have reported hyperalgesia following opioid administration, especially remifentanil, there is little known about the measures to prevent or decrease the severity or prevalence of acute OIH (7, 8). The evidence suggests that concomitant administration of low-dose naloxone can prevent the development of acute OIH, with no effect on pain management (8, 9).
2. Objectives
The current study aimed to assess the effects of intraoperative low-dose naloxone, adding to remifentanil infusion on preventing acute postoperative hyperalgesia in patients undergoing general anesthesia for laparotomy.
3. Methods
In this prospective double-blinded trial, 75 ASA I - II patients aged 18 to 75 years scheduled for an elective hysterectomy in a tertiary referral teaching hospital in Tehran from February to December 2019 were recruited. Informed written consent was obtained from all participants. Those who didn't meet inclusion criteria were excluded. Exclusion criteria were, having uncontrolled diabetes mellitus, neurologic disorders, psychologic disorder(s) which needs treatment, inflammatory and renal diseases, neuropsychiatric disorders, drug abuse, routine use or taking non-steroidal anti-inflammatory drugs (NSAIDs), opioids, or other analgesics 48 hours prior to surgery, allergy or contraindication to anesthetic agents or pain medications, including NSAIDs, acetaminophen and opioids, and history of any type of chronic pain which needed medical or interventional treatments. Patients who develop any surgical or anesthetic complication, those who needed reoperation or needed more than 2-unit transfusion of packed red blood cells, or received ketamine, antipsychotic, or gabapentinoids were considered to be excluded latter.
Patients were randomized via a computer-based method and divided into three groups each with 25 subjects, as follows: Remifentanil-Naloxone (patients receiving remifentanil 0.3 µg/kg/min and low dose naloxone 0.25 µg/kg/h in 50 mL normal saline, Remifentanil (patients receiving remifentanil 0.3 µg/kg/min), and control (patients receiving an infusion of 50 mL normal saline). All medications and placebo were administered from anesthesia induction to skin closure. The rates of injecting drugs and placebo were similar in all groups. An anesthesiologist anesthetized patients according to a written instruction; however, both patients and investigators were blinded to the intervention group and randomization process. All medications were prepared by an anesthesia nurse who was not engaged in the study.
After administering pre-medication (midazolam 0.05 mg/kg, fentanyl 2 µg/kg, and lidocaine 1 mg/kg, based on Ideal Body Weight for overweight patients) under standard monitoring, including electrocardiography (ECG), oxygen saturation (SpO2), end-tidal CO2 (ETCO2), noninvasive blood pressure monitoring (NIBP), respiratory rate (RR), and heart rate (HR), anesthesia was induced by propofol 2 mg/kg and atracurium 0.5 mg/kg. After tracheal intubation, 1.5 MAC isoflurane and muscle relaxants were used for maintaining anesthesia, if necessary. Fentanyl dose was repeated each hour to adjust the hemodynamic parameters based on anesthesia judgment.
In the remifentanil group, on the occurrence of bradycardia (HR < 50) and blood pressure drop more than 20% of baseline, interventions were provided, including administration of atropine or ephedrine and a bolus injection of crystalloids. If interventions were not efficient, the remifentanil dose was reduced. The appropriate dose of opioids was continuously adjusting based on the hemodynamic parameters during the surgery (HR and BP were increased to higher than 20% of baseline). In the post-surgery period, morphine sulfate PCA (patient-controlled analgesia) was used at 1 mg/mL; Bolus: 1 mL; lock-out interval: 7 min; basal infusion: 0 mL/h., for 24 hours after surgery to control the patient’s pain and precisely assess morphine dose consumption. All patients received IV paracetamol 1 g Q6H and IV ketorolac 30 mg Q8H.
Demographic parameters and baseline characteristics (age and surgery duration) of all participants were recorded. Total fentanyl dose used, morphine dose used in post-anesthesia care unit (PACU), morphine dose used within 24 hours after surgery, the first time-point of opioid administration after surgery and pain severity after surgery during movement, and rest according to visual analogous scale (VAS) in 0.5, 2, 6, 12, and 24 hours after surgery were documented. In PACU, shivering, the need for opioids, nausea, and vomiting (0.5, 2, 6, 12, and 24 hours after surgery) were recorded. Heart rate and blood pressure were analyzed before induction, after intubation, 30 minutes post-induction, and 30 minutes post-extubation.
The need for increasing remifentanil dose, based on hemodynamic parameters, was also documented. The severity of hyperalgesia and allodynia was assessed by static tactile tests. In this test, a soft brush that is only a sensory stimulus is contacted to the edge around the patient's wound, and in the case of allodynia, the patient drastically moves the body away from this stimulus. The severity of pain was reported using the VAS. Patients were asked to grade the highest experienced pain from one to 10 in 0.5, 2, 6, 12, and 24 hours after the surgery. A pilot study was performed on five patients from all three groups, and the results were used for calculating the sample size, by considering α = 0.5 and β = 0.2. Statistical analyses were performed using SPSS v.21.0 (IBM Corp., Armonk, NY, USA). The results are described using frequencies and mean scores. ANOVA test and Bonferroni’s post hoc test were used for comparing the groups.
4. Results
In the present study, 75 patients were separated into three groups. All participants were eligible for participation. The three groups were not significantly different concerning the baseline characteristics (age, BMI, ASA class, duration of anesthesia and surgery, and presence of underlying diseases (hypertension, asthma, and other systemic diseases) (Table 1). Hemodynamic status of patients, including HR and mean arterial pressure (MAP), were assessed at different time points. Hemodynamic parameters are described in Table 2. Except for HR in 30 minutes post-intubation and 30 minutes post-extubation, there was no significant difference between the three groups regarding the HR and MAP.
| Remifentanil-Naloxone | Remifentanil | Placebo | P-Value b | |
|---|---|---|---|---|
| Age (y) | 50.56 ± 7.0 | 50.32 ± 6.6 | 50.24 ± 5.8 | 0.7 |
| BMI (kg/m2) | 25.37 ± 4.1 | 26.04 ± 3.4 | 26.90 ± 3.4 | 0.4 |
| Surgery duration (min) | 122 ± 18 | 130 ± 24 | 128 ± 23 | 0.4 |
| Anesthesia duration (min) | 138 ± 19 | 146 ± 26 | 143 ± 25 | 0.5 |
| ASA II class (%) | 9 (36) | 8 (32) | 6 (24) | 0.6 |
| Underlying diseases (%) | 9 (36) | 8 (32) | 6 (24) | 0.6 |
Abbreviations: ASA, American Society of Anesthesiologists; BMI, body mass index.
aValues are expressed as mean ± SD unless otherwise indicated.
bOne-way ANOVA.
| Remifentanil-Naloxone | Remifentanil | Placebo | P-Value b | |
|---|---|---|---|---|
| HR at baseline | 85.1 ± 9.4 | 82.1 ± 13.1 | 81.4 ± 13.1 | 0.89 |
| HR after intubation | 91.9 ± 9.4 | 95.1 ± 14.5 | 98.6 ± 14.8 | 0.25 |
| HR at 30 mins after intubation | 60.6 ± 11.1 | 59.1 ± 9.5 | 87.2 ± 12.3 | 0.001 |
| HR after extubation | 75.2 ± 9.4 | 72.5 ± 9 | 94.6 ± 11.9 | 0.001 |
| MAP at baseline (mmHg) | 79.5 ± 6.7 | 79.1 ± 6.5 | 78.1 ± 6.2 | 0.83 |
| MAP after intubation (mmHg) | 94.3 ± 6.1 | 93.5 ± 6.9 | 92.8 ± 5.7 | 0.74 |
| MAP at 30 mins after intubation (mmHg) | 73.9 ± 6.2 | 72.7 ± 5.5 | 71.3 ± 5.6 | 0.65 |
| MAP after extubation (mmHg) | 84.6 ± 6.6 | 83.1 ± 6.2 | 84.7 ± 6.8 | 0.55 |
Abbreviations: HR, heart rate; MAP, mean arterial pressure.
aValues are expressed as mean ± SD.
bOne-way ANOVA.
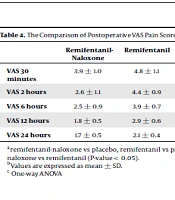
Comparing EtCO2 and SpO2 in PACU revealed no significant difference in various time points between the three groups. The three groups were compared concerning intraoperative fentanyl dose, time of the first dose of rescue analgesia in recovery, rescue morphine dose in the recovery room, and total 24-hours postoperative morphine dose (Table 3). Cumulative intraoperative fentanyl dose was significantly higher in the control group than the other two groups (P-value = 0.001). The time of the first dose of rescue analgesia in recovery was significantly earlier in the remifentanil group compared to the control and remifentanil-naloxone groups. Also, this time was significantly earlier in the control group than the remifentanil-naloxone group (P-value = 0.001). The dose of rescue morphine sulfate in the remifentanil group was significantly higher than the two other groups (P-value = 0.002). Also, 24-hours postoperative morphine sulfate consumption was significantly higher in the remifentanil group compared to other groups (P-value = 0.001). The need for atropine was not significantly different between the three groups (P-value = 0.810), but the incidence of shivering in PACU was significantly lower in the remifentanil-naloxone group (8%) compared to the remifentanil (40%) and control (20%) groups (P-value = 0.02). The incidence of nausea and vomiting after surgery in the remifentanil-naloxone, remifentanil, and control groups was 28%, 32%, and 32%, respectively (P-value = 0.922). Pain severity was assessed postoperatively in 5-time points, which was significantly lower in the remifentanil-naloxone group compared to the other two groups. The details of postoperative pain, measured by VAS, are provided in Table 4.
| Remifentanil-Naloxone | Remifentanil | Placebo | P-Value b | |
|---|---|---|---|---|
| Cumulative intraoperative fentanyl (µg) | 76 ± 38 | 90 ± 35 | 160 ± 47 | 0.001 |
| Time of first dose of rescue analgesia in recovery (min) | 41 ± 6 | 26 ± 4 | 33 ± 4 | 0.001 |
| Rescue morphine sulphate (mg) | 8.2 ± 3.5 | 12.1 ± 4.7 | 9.1 ± 3.3 | 0.002 |
| 24-hours postoperative morphine sulphate (mg) | 27.7 ± 5.1 | 39.1 ± 7.8 | 36.1 ± 2.8 | 0.001 |
aValues are expressed as Mean ± SD unless otherwise indicated.
bOne-way ANOVA
| Remifentanil-Naloxone | Remifentanil | Placebo | P-Value c | |
|---|---|---|---|---|
| VAS 30 minutes | 3.9 ± 1.0 | 4.8 ± 1.1 | 5.0 ± 1.5 | 0.013 |
| VAS 2 hours | 2.6 ± 1.1 | 4.4 ± 0.9 | 3.4 ± 1.0 | < 0.001 |
| VAS 6 hours | 2.5 ± 0.9 | 3.9 ± 0.7 | 3.3 ± 0.6 | < 0.001 |
| VAS 12 hours | 1.8 ± 0.5 | 2.9 ± 0.6 | 2.4 ± 0.4 | < 0.001 |
| VAS 24 hours | 1.7 ± 0.5 | 2.1 ± 0.4 | 2.2 ± 0.5 | 0.015 |
aremifentanil-naloxone vs placebo, remifentanil vs placebo and remifentanil-naloxone vs remifentanil (P-value < 0.05).
bValues are expressed as mean ± SD.
c One-way ANOVA
Assessment of hyperalgesia with tactile test revealed a higher incidence of hyperalgesia in the remifentanil group in 0.5, 2, 6, 12, and 24 hours after surgery compared to the other two groups, which was statistically significant at 0.5, 2, and 6 hours after surgery (P < 0.05). The details of the test at different time points are shown in Table 5.
| Remifentanil-Naloxone, No. (%) | Remifentanil, No. (%) | Control, No. (%) | P-Value a | |
|---|---|---|---|---|
| Positive test after 30 minutes | 4 (16) | 13 (52) | 8 (32) | 0.02 |
| Positive test after 2 hours | 3 (12) | 10 (40) | 4 (16) | 0.03 |
| Positive test after 6 hours | 2 (8) | 7 (28) | 1 (4) | 0.03 |
| Positive test after 12 hours | 2 (8) | 5 (20) | 0 | 0.06 |
| Positive test after 24 hours | 0 | 2 (8) | 0 | 0.32 |
aOne-way ANOVA.
5. Discussion
This study demonstrated that adding ultra-low doses of naloxone can improve analgesia during the postoperative period after intraoperative remifentanil infusion. Patients in the remifentanil group received significantly higher amounts of opioids and had higher scores of hyperalgesia and pain intensity compared to the other two groups. A few mechanisms, including opioid-induced hyperalgesia, tolerance, and withdrawal syndrome, have been proposed for increased pain intensity after opioids infusion. The pain resulting from tolerance to opioids usually happens in the site of tissue injury and relieved by additional opioids. OIH will be perceived even in the distant areas (far from the tissue injury site), almost everywhere in the body. The intensity of the pain caused by the OIH will be increased if additional opioids be administered. OIH can be managed by reducing opioid doses, opioid rotation, or anti-N-methyl-D-aspartate (NMDA) receptor medications such as ketamine (6). However, abrupt cessation of the remifentanil infusion may cause acute withdrawal syndrome. Several mechanisms contribute to the appearance of OIH. These mechanisms include central sensitization due to suppressed reuptake or the increased release of excitatory neurotransmitters (glutamate and aspartate), downregulation of µ-opioid receptors in the periaqueductal gray, enhancement of nociceptive responses, and genetic contributions. (6, 8). However, few studies have investigated these mechanisms.
A review by Kim et al. (2014) (8) reported that clinical studies support the development of OIH in healthy subjects and patients after administration of short-acting opioids, such as remifentanil, with an infusion rate of > 0.1 μg/kg/min. The findings also revealed that remifentanil infusion with a dose of 0.3 μg/kg/min leads to OIH. In the present study, hyperalgesia was confirmed by the presence of the following signs: development of allodynia (assessed by brush test with more frequent positive results), the need for rescue analgesia in PACU (both required dose and the time of need to the first dose), total 24-hours postoperative morphine dose used, and the severity of pain (measured using the VAS). In the present study, patients in the remifentanil-naloxone group showed less severe pain and had lower analgesic requirements in PACU and 24 hours after surgery compared to the remifentanil and placebo groups. Intraoperative fentanyl requirement also was significantly lower in the remifentanil-naloxone group. Naloxone is an agent with a biphasic impact on pain. A low dose of naloxone can enhance the analgesic effect of opioids, but higher doses would reverse analgesia induced by opioids (10).
The precise mechanism of low-dose naloxone is not identified yet, but its anti-hyperalgesic properties are caused by antagonizing or modifying NMDA, and μ-opioid receptor activities related to the opioid-induced hyperalgesia are considering as major contributors (9). Movafegh et al. also reported that infusion of low-dose naloxone was associated with lower postoperative morphine doses in patients undergoing hysterectomy (11). In addition to improving the analgesia, in the remifentanil group, low-dose naloxone could prevent OIH. Morphine is a derivate of opium, which is a narcotic with potent analgesic effects. Analgesic actions of morphine can be attenuated by naloxone, which is an opioid antagonist. Naloxone competitively attacks opioid receptors (12-14). This antagonist is widely using for managing morphine abuse or overuse-related complications. The and K receptors are mediators for analgesic effects, but receptor does not have an analgesic effect and causes dysphoria, delusion, and respiratory agitation. Naloxone can block the receptors of opioids in the spine and higher nervous levels, which eventually causes hyperalgesia (15-18). Most of the studies in this arena have investigated the hyperalgesia caused by chronic administration of opioids, and evidence regarding the acute OIH are scarce (19-22).
Hoshijima et al. revealed that remifentanil was significantly associated with higher rates of postoperative shivering compared to alfentanil and fentanyl, but its effects were similar to sufentanil (23). Studies have shown that naloxone contains anti-shivering properties (24, 25). The present study also showed that post-surgery shivering was significantly more frequent in the remifentanil group. Some evidence supports the idea that postoperative shivering after administration of remifentanil may be a sign of acute opioid withdrawal. Rapid clearance of remifentanil by non-specific esterases leads to the lowest CSHT (ultra-short context-sensitive half-time) for remifentanil among all opioids, which may be a major underlying mechanism for shivering after surgery. Hoshijima et al. showed that patients in both groups of low-dose and high-dose remifentanil experienced shivering, but its frequency was higher in high-dose cases (23). In the present study, shivering was more common in the high-dose remifentanil (without naloxone) group. Our study also showed that the remifentanil (with low-dose naloxone) was associated with a significantly lower rate of postoperative shivering compared to the remifentanil and placebo. It seems that the observed decline in the shivering rate can be attributed to the anti-acute opioid withdrawal effect of naloxone.
Another study has shown that pain rating during meditation was significantly lower among those who received naloxone than saline and concluded that naloxone's ability to block opioid receptors can enhance meditation analgesia (26). This issue can be attributed to the paradoxical effect of naloxone, which can be summarized as enhancement in the release of endogenous opioids and up-regulation of opioid receptors. In the present study, it was also observed that the ultra-low dose of naloxone led to significantly lower doses of 24-hours morphine consumption in recovery compared to the other two groups.
The main limitation of the present study was not using appropriate devices to assess allodynia and hyperalgesia (e.g., von Frey hair kit), which may have affected the precision of hyperalgesia and allodynia evaluation. Moreover, we could not differentiate OIH from acute tolerance. Therefore, the improved analgesic effect of naloxone in this study can be attributed to two issues: low dose naloxone has an analgesic effect, or it can reduce the OIH.
5.1. Conclusion
This study demonstrated the efficacy of intraoperative low-dose naloxone (0.25 μg/kg /h) added to remifentanil infusion on reducing the frequency and severity of acute postoperative hyperalgesia in patients undergoing general anesthesia for laparotomic hysterectomy.