1. Background
Epilepsy is a disorder that affects 1% of the global population. It is the second most common serious neurological disorder after stroke, affecting humans (1, 2). About 50 million individuals have epilepsy worldwide, and 90% of them come from developing nations. It is a widespread progressive neurological condition in which unregulated excitability and recurring unprovoked seizures define the equilibrium between cortical excitability and inhibition (3-6). There is no conclusive indication that the pathophysiology and effects of seizures have distinct distinctions between immature and adult brains. It is a series of multiple types of seizures that differ greatly in severity, appearance, origin, effect, and management (7-9).
Drugs are primary epilepsy treatment, but 60 - 90% of patients with epilepsy can be managed by proper selection and application of antiepileptic drugs (AEDs). The use of an effective seizure control drug depends on seizure diagnosis, patient compliance, and drug side effects, which play an important role in patient compliance (10-15). Since antiepileptic medications have a limited therapeutic index and any organ may be impaired by their adverse effects, their extensive use has substantial safety consequences. Overall, because of intolerance, 10 - 30% of individuals with epilepsy discontinue their originally recommended antiepileptic medicine. The prevalence of adverse effects ranges between 10% and 40% for patients chronically infected with antiepileptic medications. It is also important for optimal clinical practice to consider the manifestations of opioid toxicity, risk factors, and appropriate preventive steps (16, 17).
Pharmacovigilance is important for the safety of public health because adverse reactions to pharmaceutical drugs for human consumption are avoided, identified, and measured. This includes the administration of pharmaceutical items for human consumption during the life cycle, keeping in view human safety (18-21). As a result, we must highlight the need for pharmacovigilance as continuity and completion of the study of pharmaceutical products starting from clinical trials. The risks posed by the ever-increasing number of drugs, each of which carries an inherent risk of unforeseeable potential for injury, continue to play an important role in resolving them. Whenever adverse effects and toxicity arise, particularly when previously unknown, they must be identified, evaluated, and their importance accurately conveyed to people who know how to perceive the facts (22-27). By ensuring that pharmaceutical products of high consistency, purity, and effectiveness are used rationally, damage can be minimized. We must ensure that the risk of opioid use is expected, well-handled, and conveyed to regulatory agencies and other healthcare providers to accomplish this purpose and increase a sense of trust among patients (28-31). Various Adverse Drug Effects (ADRs) are seen due to the longtime of epilepsy therapy, changing of dosage, and supervision (30, 32-35).
The accuracy and reliability of medication outcome measures are boosted by randomized controlled trials, but specific clinical safety data are not available. Nevertheless, for different ethical, statistical, and practical reasons, the organization of regulated epidemiological practice, which is inclined to provide comprehensive information on ADRs, is exceptionally hard. In India, AED safety monitoring relies primarily on the introduction of the national ADR reporting system, which is a system of spontaneous reporting (SR). However, the challenges of underreporting and flawed data are still hard to address in the SR method. The shortcomings of the SR method may be compensated for by active supervision by physicians, but such study is comparatively lacking. We performed a clinical, observational study to assess ADRs associated with antiepileptic drugs to fill this void. This study focused on the chance of ADR development in patients with epilepsy.
2. Objectives
This study was conducted to assess ADRs arising due to antiepileptic drugs in the Neurology Department of a Tertiary Care Hospital, Srinagar, Jammu & Kashmir, India.
3. Methods
This study was conducted at the Neurology Department of a Tertiary Care Hospital, Srinagar, Jammu & Kashmir, India, for eight months. It was a prospective observational study. Spontaneous reports of ADRs by practicing physicians in the outpatient and inpatient settings of the hospital were included in the study. This study was approved by the Institutional Ethics Committee of the University of Kashmir. The dependent (outcome) variable was the prevalence of ADRs due to antiepileptic drugs. The data interpretation was based on descriptive statistics. The data obtained were presented as mean± Standard Mean Error (SEM) and, where applicable, as percentages. Using MS Excel and SPSS predictive packages of version 20, drug data and patients’ characteristics were computed. For the evaluation of the relationship between variables, sufficient statistical tests were used.
4. Results
4.1. Epidemiology of Adverse Drug Reaction
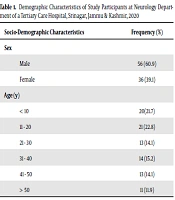
Of 3,300 patients who were on AED therapy during the study period, 92 patients had AED-related ADRs, with a prevalence of 3.07%. Out of 92 patients developing ADRs, 56 were males, and 36 were females. A total of 18 cases were reported in the inpatient department and 74 cases in the outpatient setting. About 51 of the patients visited the hospital due to ADRs, while the rest of 41 patients were detected during their regular follow-ups in the OPD setting. Six age ranges were listed as patients with ADRs. In the age range of 11 - 20 years, the frequency of ADRs was observed to be greater, and the minimum was > 50 years (Table 1).
| Socio-Demographic Characteristics | Frequency (%) |
|---|---|
| Sex | |
| Male | 56 (60.9) |
| Female | 36 (39.1) |
| Age (y) | |
| < 10 | 20 (21.7) |
| 11 - 20 | 21 (22.8) |
| 21 - 30 | 13 (14.1) |
| 31 - 40 | 14 (15.2) |
| 41 - 50 | 13 (14.1) |
| > 50 | 11 (11.9) |
4.2. Types of Adverse Drug Reactions, Common Drugs, and Management of Adverse Drug Reactions
Antiepileptic drugs used for various neurological disorders can cause different types of ADRs, but the most commonly occurring ADRs were loss of appetite, skin rashes, gum hypertrophy, tremors, and others (dizziness, weight gain, nausea, vomiting). Out of 80 ADRs, 42.5% were related to valproate, followed by phenytoin, carbamazepine, levetiracetam, and others (pregabalin, oxcarbazepine). The ADRs that occurred in the Neurology Ward were managed using different measures. In 22 patients, therapy with the suspected drug was changed. In 15 patients, the dose of the drug was reduced in therapy with suspected AED (Table 2).
| Variables | No. (%) |
|---|---|
| Types of adverse drug reaction | |
| Loss of appetite | 32 (34.78) |
| Skin rash | 16 (17.39) |
| Gum hypertrophy | 9 (9.78) |
| Tremors | 9 (9.78) |
| Nausea and vomiting | 06 (6.52) |
| Abdominal pain | 05 (5.43) |
| Diarrhea | 0.3 (3.26) |
| Stomach pain | 0.3 (3.26) |
| Increase in pulse | 0.3 (3.26) |
| Headache | 0.3 (3.26) |
| Othersa | 0.3 (3.26) |
| Common drugs | |
| Carbamazepine | 18 (19.56) |
| Sodium Valproate | 38 (41.30) |
| Phenytoin | 21 (22.82) |
| Levetiracetam | 8 (8.69) |
| Othersb | 7 (7.60) |
| Measures for management | |
| Drug changed | 22 (23.91) |
| Dose reduced | 15 (16.30) |
| Doses reduced and another drug added | 15 (16.30) |
| No change | 17 (18.47) |
| No change and other drug added | 12 (13.04) |
| Drug withdrawn | 11 (11.95) |
aBlack stool, hot flushes
bClobazam, diazepam, divalproex, ethosuximide, gabapentin, pregabalin, and vigabatrin
4.3. Causality Assessment and Severity Assessment of Adverse Drug Reactions
Causality assessment was done using the Naranjo scale, and according to the score, the ADRs were classified as “definite/highly probable”, “probable”, “possible”, or “unlikely”. Out of 92 ADRs, 42 (45.65%) ADRs were identified as possible, followed by 40 (43.47%) as probable and 10 (10.86%) as definite.
The severity of the patients with ADRs was analyzed using the Hartwig scale (36), and accordingly, they were grouped as "mild", "moderate", or “severe”. Most of the patients were classified as mild (n = 49; 53.26%) and moderate (n = 43; 46.73%) patients. No patients were found to be “severe”. No ADR was recorded that caused permanent harm or led to the death of the patient.
5. Discussion
In this study, the prevalence of ADRs among patients taking AED therapy was below 5%. The study showed that ADRs were most common in the 11 - 20 age group. This result was contrary to the findings from many other studies where children and the elderly were shown to be more prone to developing ADRs (37-39). The low incidence of ADRs among the extreme age groups in our study might be due to the special considerations taken by the practicing physicians in the Neurology Ward concerning prescribing and titrating doses in these vulnerable age groups to prevent avoidable ADRs.
The high number of ADRs reported among 11 - 20 years of age might be due to the changing hormonal milieu in adolescents, which affects the drug metabolism and predisposes ADRs in this age group. In our study, ADRs were found to be more frequent in males (56 patients) than in females (36 patients). Compared to female patients, this might be due to the large number of male patients attending the ward. The most offending drug was found to be sodium valproate, accounting for around 41.30% of the overall prescribed medications that triggered ADRs, followed by the prescription of phenytoin, carbamazepine, and levetiracetam. These findings were in line with previously published studies. (40-43). The most reported ADRs in this study were appetite loss, followed by giddiness/nausea and vomiting. The hepatotoxicity associated with valproate is well known in the literature. The first symptom of irregular functioning of the liver is anorexia. In both, sodium valproate was found to be the offending drug. Phenytoin is the only drug that caused gum hypertrophy, and the majority of the drugs caused skin rashes.
5.1. Conclusions
Still, the prevalence of ADR due to the antiepileptic drug is significant. For the early diagnosis and avoidance of ADRs, frequent follow-ups of patients on AEDs are needed to improve patient compliance with drug therapy and provide better drug therapy for avoiding associated morbidity and mortality. For this approach to succeed, a “therapeutic alliance” between the patient and clinician is essential. Based on the pharmacology of the AEDs used, medication reactions capable of delivering potentially life-threatening outcomes should be scientifically expected in patients needing AEDs. It can help reduce drug interactions and AEs by reducing polytherapy and choosing AEDs with desirable pharmacokinetic profiles. The tendency to produce these reactions may be affected by various endogenous and environmental influences. The likelihood of early diagnosis and care can be improved by a high degree of suspicion, information about risk factors, and strong physician-patient contact. It is important to thoroughly register and report the diagnosis of serious reactions to the health authorities. The extremely unusual incidence of life-threatening incidents never limits decision-making on care. Future studies into epidemiology, chemistry, and genetics may include methods for assessing which patients are at risk, so excessive exposure should be avoided. The information was beneficial to detect ways to fix issues and also to figure out how to treat patients whenever adverse reactions arise such that the outcomes can be applied to future patient care practices.