1. Context
Parkinson’s disease (PD) is the second most common neurodegenerative disorder, which is commonly characterized by resting tremor, bradykinesia, rigidity, gait disturbances, and postural instability. About one to three percent of people aged 65 years or older are affected by PD. Although the pathogenesis of PD is not fully clarified, the degeneration of dopaminergic neurons of the substantia nigra, locus coeueus, and other brain stem dopaminergic cell groups, with the progressive loss of dopaminergic neurons terminals in the striatum, is responsible for most of the weakening features of the disease (1, 2) It is thought that multiple genetic and environmental factors, either as risk or protective factors, have a role in the development and progression of the disease (3, 4).
Caffeinated beverages, particularly coffee and tea, are the most popular and commonly consumed beverages worldwide (5). Although several studies have shown a dose-dependent inverse relationship between caffeine consumption and the risk of developing PD, more research still seems necessary. The systematic review and meta-analysis of observational studies have demonstrated a 25 - 30% reduction in the risk of PD among caffeine consumers, and there is a linear dose-response relation; the higher is the consumption of caffeine, the lower is the risk of PD (6-8). Caffeine is a nonselective competitive blockade of adenosine receptors, especially adenosine A1 receptors and A2A receptors, and it has stimulant effects on locomotion by the A2A receptor blockade (9, 10). Caffeine, as an antagonist of adenosine A2a receptors, has been shown to have a protective role against PD in animal models (11).
Given studies about caffeine effects, it seems that caffeine has neuroprotective effects on the progression of PD symptoms. Several clinical trials have indicated conflicting findings of the association of caffeine and the onset or progression of PD symptoms, especially motor symptoms. Therefore, it is still unclear whether caffeine consumption or supplementation can positively or negatively affect PD symptoms. Moreover, to the best of our knowledge, there is no published systematic review about the effects of caffeine consumption on the symptoms of PD. Additionally, most of the performed systematic reviews have focused on the association of caffeine and the risk of PD, mostly performed with observational studies.
2. Objectives
We conducted a systematic review of clinical trials to quantify the association between caffeine intake and the progression of PD symptoms.
3. Methods
3.1. Search Strategy and Selection Procedures
Randomized controlled trials (RCTs), crossover studies, and quasi-experimental studies were assessed to evaluate the effect of caffeine on PD. The databases, including Medline/PubMed, Embase, ProQuest, Cochrane Library, and ClinicalTrials.gov were systematically searched until August 2020 without a restriction of publication date. The databases, including Web of Science and Google Scholar were also evaluated manually. The MeSH terms, as well as free keywords, were used for the search. Based on the searched databases, the following keywords were used singly or cross-linked: ‘Parkinson’s disease’, ‘Parkinson’, ‘Parkinsonism’, ‘caffeine’, ‘coffee’, and ‘tea’. The results were presented following the PRISMA guidelines.
3.2. Inclusion and Exclusion Criteria
3.2.1. Studies
In this study, all types of clinical trials including RCT, crossover, before-and-after, and quasi-experimental studies evaluating the effects of caffeine on PD were included. Only full-text articles published in the English language were evaluated. Books, book chapters, reviews, letters, short surveys, abstracts, conference abstracts, commentaries, studies based on the description of a protocol or perspective of the authors, and comments on an article were excluded.
3.2.2. Participants
Study participants were patients with idiopathic PD stage I - IV (Hoehn and Yahr Scale). Patients with daily caffeine intake > 150 mg assessed by a standardized intake questionnaire (12), supraventricular cardiac arrhythmia, uncontrolled hypertension, malignancy, active peptic ulcer disease, premenopausal women not using birth control, other progressive neurological disorder, patients with migraine or other headache types related to the consumption of coffee, clinically significant psychiatric illnesses or psychotic symptoms, dementia Folstein Mini-Mental State Examination < 23/30 with consequent activities of daily living impairment, Beck Depression Inventory scores ≥ 15, history of alcohol abuse or other substance abuse within the past two years, severe intolerance or allergy to caffeine, another untreated reversible cause of EDS, use of prescribed alerting agents, another adenosine antagonist, lithium or clozapine, and changes to antiparkinsonian medication in the last three months were excluded from the study.
3.3. Types of Interventions and Comparison
In all studies, interventions included caffeine as dose-escalation, which was started with 200 mg/day and increased during the study until the patient tolerated it. The comparison group received a placebo or no intervention.
3.4. Outcome Measures
The primary outcome measure was the Unified Parkinson’s Disease Rating Scale (UPDRS I–IV) (13), and the Epworth Sleepiness Scale (ESS) scores (14). The secondary outcome measure was the maximally tolerated dose, which was defined as the last dose before experiencing a limiting adverse effect.
3.5. Literature Quality Assessment
Two authors (AR and EF) independently evaluated the acceptability of the papers. In the case of disagreement between the authors, the study was first discussed, and if the disagreement persisted, the final decision was made consulting with the third author (SS). The Cochrane Collaboration’s tool for assessing the risk of bias in randomized clinical trials was used for assessing the quality of RCTs (15). This is a standard tool that evaluates the methodological quality of clinical trials for detecting any bias such as selection bias, performance bias, detection bias, attrition bias, reporting, and other biases.
The quality of non-randomized studies was assessed using the Cochrane risk of bias assessment tool for non-randomized studies of interventions (ROBINS-I) (16), which assesses the risk of bias in seven domains: (1) bias due to confounding; (2) bias in participant selection; (3) bias in classification of interventions; (4) bias due to deviations from intended interventions; (5) bias due to missing data; (6) bias in the measurement of outcomes; and (7) bias in the selection of reported results. To determine the risk of bias in RCTs, Review Manager Software, version 5.3 (The Nordic Cochrane Centre, Copenhagen, Denmark) was used.
3.6. Data Extraction
The guidelines of the Cochrane handbook for systematic reviews of interventions were used to design the data collection form (17). The information included the name of the authors, publication year, study type, sample size, age of participants, intervention, comparison group, follow-up duration, outcomes, outcome measurement, results, and side effects. The corresponding author of the original study was contacted via email in case of incomplete or ambiguous data.
4. Results
4.1. Study Characteristics
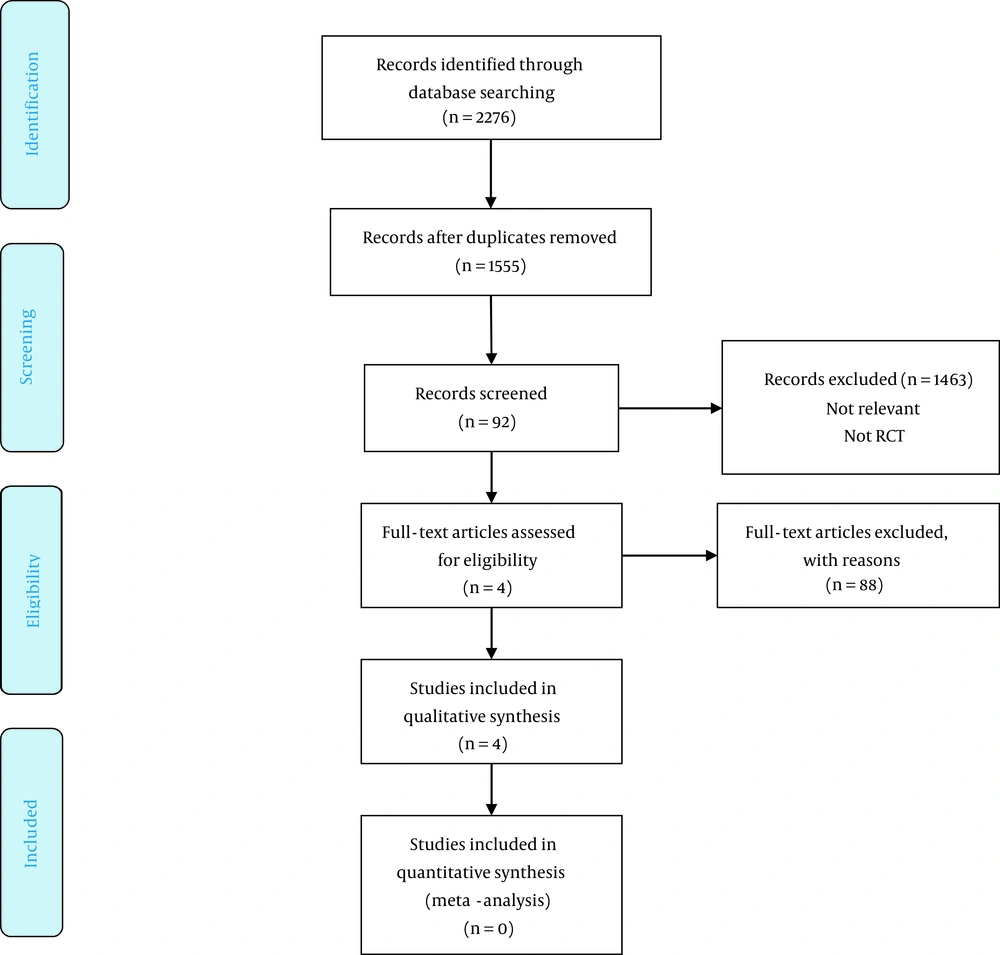
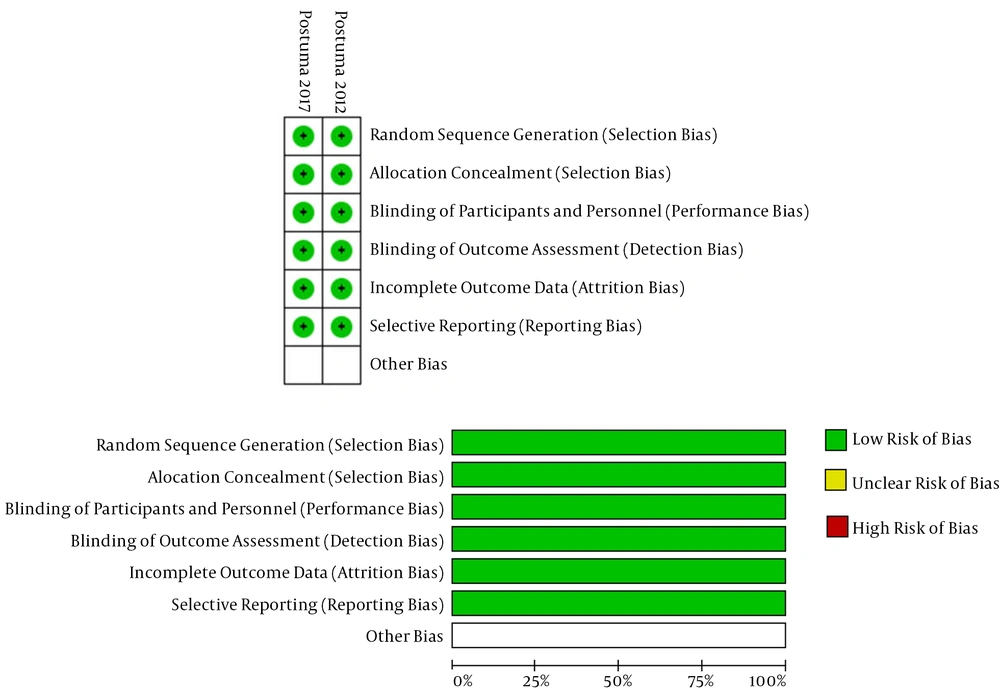
The database search resulted in 2,276 papers, of which 1,555 papers remained after removing duplicates. After a review of their titles and abstracts, 1,463 records were excluded because they were inappropriate for inclusion. The full texts of the remaining 92 potentially related studies were obtained. Eighty-eight studies were excluded because of not meeting the inclusion criteria, and the remaining four papers (18-21) were included in the study. The PRISMA flowchart of the study is shown in Figure 1. Table 1 shows the general characteristics of each study. Table 2 and Figures 2A and B show the risk of bias in RCTs, and Table 3 shows the risk of bias in non-randomized studies.
| References | Type of Clinical Trial | Sample Size | Age of Participants (Mean ± SD) | Intervention (Dosage and Duration of Treatment ) | Comparison (Dosage and Duration of Treatment ) | Duration of Follow-up | Outcome | Outcome Measures | Results | Adverse Events |
|---|---|---|---|---|---|---|---|---|---|---|
| Altman et al. (2011) (18) | Pilot open-label | Caffeine group (n = 25) | 65.5 ± 9 | Dose started at 100 mg BID and was escalated weekly in 200-mg increments until week 5. For weeks 5 and 6, the dose remained at 1,000 mg QD | - | Unified Parkinson’s Disease Rating Scale (UPDRS I-IV/); Epworth Sleepiness (ESS); Parkinson’s Disease Questionnaire (PDQ); Pittsburgh Sleep Quality Index (PSQI); Timed ‘‘Up and Go’’ (seconds); PSQI overall; Beck Depression Inventory; Beck Anxiety Inventory; Fatigue Severity Score | Unified Parkinson’s Disease; Rating Scale (UPDRS I-IV/); Epworth Sleepiness Scale (ESS) | Caffeine had significant effects on ESS, UPDRS, PDQ-39, and PSQI; No significant changes in anxiety and depression score; Improvement in clinical global overall | Nausea, dyspepsia, and malaise; Worsening parkinsonian and emerging or enhanced physiologic tremors, anxiety, and insomnia | |
| Ferreira et al. (2016) (20) | n-of-1 Trial (multiple single-patient clinical trials) (crossover) | Caffeine group (n = 4) | - | Unified Parkinson’s Disease Rating Scale (UPDRS I-IV/); Epworth Sleepiness; Daytime somnolence; Somnolence during tasks | Unified Parkinson’s Disease; Rating Scale (UPDRS I-IV/); Epworth Sleepiness Scale (ESS), 7-point Likert scale monitoring subjective;7-point scale monitoring subjective | Caffeine affected ESS; no trend was captured; the findings of UPDRS indicated no parkinsonism aggravation | No adverse event | |||
| Postuma et al. (2012) (21) | Randomized controlled trial (parallel) | Caffeine group (n = 30); placebo group (n = 31) | Caffeine group: 65.2 ± 8.3; Placebo group: 62.4 ± 7.5 | 100 mg twice daily ×3 weeks, then 200 mg twice daily ×3 weeks or 6 weeks | Matching placebo in 1:1 ratio | - | Epworth Sleepiness Scale; Unified Parkinson’s Disease; Rating Scale (UPDRS I-IV/); Clinical Global Impression of Change (CGI-C); Fatigue Severity Scale (FSS); Pittsburgh Sleep Quality Index; Beck Depression Inventory; Parkinson’s Disease Questionnaire–39; Short Form–36 (SF-36), Quality of Life Scale | Unified Parkinson’s Disease Rating Scale (UPDRS I-IV/), Epworth Sleepiness Scale (ESS) | Caffeine reduced UPDRS score in 3 weeks but significant changes were observed in 6 weeks. As compared with placebo after the exclusion of the 4 protocol violations, there was a significant reduction in ESS score in 6 weeks. no changes were observed in PSQI, FSS, and PDQ-39. An improvement was seen in the general health; Component of the SF-36. | No adverse event |
| Postuma et al. (2017) (19) | Randomized controlled trial (parallel) | Caffeine group (n = 60); placebo group (n = 61) | Caffeine group: 62.4 ± 7.5; Placebo group: 62.3 ± 8.4 | 200 mg twice daily (the dose was increased slowly) (50 mg per week), with placebo for the first week, and full dose reached in week 9 | Matching placebo in 1:1 ratio | 18 months | Movement Disorder Society–sponsored Unified Parkinson’s Disease Rating Scale (MDS-UPDRS), Epworth Sleepiness Scale, Quality of life; Montreal Cognitive Assessment (MoCA) | Motor severity, assessed with the Unified Parkinson’s Disease; Rating Scale (UPDRS); the 5-item EuroQoL 5-dimension | No significant effect of caffeine on UPDRS was observed. Caffeine had a small effect on MDS-UPDRS and points on SCOPA–sleep nighttime), and MoCA. | No adverse event |
| Bias Risk | Postuma et al. 2012 (21) | Postuma et al. 2017 (19) |
|---|---|---|
| Random sequence generation (selection bias) | Y | Y |
| Allocation concealment (selection bias) | Y | Y |
| Blinding of participants and personnel (performance bias) | Y | Y |
| Blinding of outcome assessment (detection bias) | Y | Y |
| Incomplete outcome data (attrition bias) | Y | Y |
| Selective reporting (reporting bias) | Y | Y |
Abbreviations: N, no (i.e., high risk of bias); Y, yes (i.e., low risk of bias).
4.2. Description of Studies
The total number of participants in the four included studies was 207. Sample sizes varied from 25 to 61 participants. Of the four included studies, two were RCTs (19, 21), and two were non-randomized single-arm studies (18, 20). In these four studies, caffeine was evaluated separately and was compared with placebo in the RCTs (19, 21).
The treatment duration in studies varied from five days to 18 months (18-21). The primary outcome of all studies was the effect of caffeine on ESS and UPDRS. The secondary outcome was the tolerable dose of caffeine in PD patients. All studies evaluated the effect of caffeine in PD patients as dose-escalation, which is explained in detail in Table 1.
In all studies, ESS and UPDRS were evaluated by the same methods (13, 14). The findings of two studies were presented as the mean ± SD and the other ones reported as the mean and 95 or 80% confidence interval. Since the duration of intervention was very different from five days to 18 months and the number of studies was low, it was not possible to combine the data and conduct a meta-analysis.
4.3. Methodological Quality
Among the included papers in the study, two studies were quasi-experimental single-arm clinical trials (18, 20), one of which was an open-label study (18), and the other one was an n-of-1 trial (20). The others were RCTs and used sequence allocation using a computer program and a random number table. Blinding was described in both studies, which were double-blinded.
In all studies, participant withdrawal was reported, but only two studies included an intention-to-treat analysis (19, 21). In the n-of-1 trial, the final analysis was performed on patients who completed the study (20).
The protocols of the two RCTs were available (19, 21), and therefore considered to have a low selective reporting bias (Table 2).
To assess the quality of non-randomized studies, the ROBINS-I (16) was used, which evaluated the risk of bias in seven domains. Both studies were at low risk of bias in the selection of participants, bias in the classification of interventions, and bias due to deviations from intended interventions (Table 3).
| Variables | Altman et al. 2011 (18) | Ferreira et al. 2016 (20) |
|---|---|---|
| Bias due to confounding | Low | Serious |
| Bias in selection of participants | Low | Low |
| Bias in classification of interventions | Low | Low |
| Bias due to deviations from intended interventions | Low | Low |
| Bias due to missing data | Serious | Serious |
| Bias in measurement of outcomes | Serious | Low |
| Bias in selection of reported result | No information | No information |
| Overall | Low | Low |
Abbreviations: Low, low risk of bias (the study is comparable to a well-performed randomized trial with regard to this domain); No information, no information on which to base a judgment about risk of bias for this domain; Serious, serious risk of bias (the study has some important problems).
4.4. The Effect of Caffeine on ESS
Of four studies, only one study reported the significant effect of caffeine on ESS (18). One study noted that no trend was captured (20). In the other study, no significant effect of caffeine on ESS was reported. Postuma et al. (21) observed no significant effects of caffeine on ESS during three- and six-week interventions. However, they reported a significant reduction in ESS scores in the sixth week after excluding the four protocol violations.
4.5. The Effect of Caffeine on UPDRS
Of all the included studies, one study reported the significant effect of caffeine on UPDRS (18). In one study, the baseline score of UPDRS was noted, but there was no report about the changes of UPDRS after the intervention; they reported that the findings of UPDRS indicated no Parkinsonism aggravation. The other one reported no significant effect of caffeine on UPDRS. Postuma et al. (21) reported that the UPDRS score reduced in the third week, but significant changes were observed after six weeks.
4.6. Comparison Between Caffeine and Placebo and Their Effects on ESS and UPDRS
One of the RCTs reported no significant effect of caffeine on ESS and UPDRS as compared with placebo (21). In comparison with placebo, the other one reported that caffeine reduced UPDRS after three weeks, but this change was significant after a six-week intervention. Moreover, after excluding the four protocol violations, there was a significant reduction in ESS scores in the sixth week.
4.7. Side Effects
Side effects were noted in all the articles included in the study. In RCTs, no serious adverse effects of caffeine were reported. Also, in the n-of-1 trial, no adverse effect of caffeine was reported (20). In the open-label study, adverse effects of caffeine were reported and noted; only three participants out of 25 patients completed the study and the remaining subjects withdrew from the study because of adverse effects (18). Major complaints of the patients were nausea, dyspepsia, and malaise.
5. Discussion
5.1. Findings and Interpretation
Parkinson’s disease is the second most common neurological disorder (1). Various case-control and cohort studies have suggested a large number of environmental factors for predicting the risk of PD (8, 22), of which caffeine intake is one of the most well-established protective factors (6, 23-27).
Although caffeine is known to be an antagonist of adenosine A2a receptors, it has been suggested to act as an A2a inverse agonist (28). Caffeine and other more specific A2a receptor antagonists are shown to have neuroprotective effects in animal models of PD (29).
These data have led to the hypothesis that caffeine may have a neuroprotective effect in PD. Therefore, there are many studies evaluating the effect of caffeine consumption/supplementation on PD progression, movement, sleep disorders, etc. The findings of these studies are inconclusive, and it cannot be concluded if caffeine consumption can improve or worsen the symptoms. Thus, we reviewed the published article about the effects of caffeine supplementation on ESS and UPDRS.
The present review of clinical trials indicated that caffeine did not affect ESS and UPDRS in PD. Of four eligible articles included in this study, only one study reported the significant effect of caffeine on ESS and UPDRS. In the study by Postuma et al. (21), the significant effect of caffeine on UPDRS was observed after six weeks.
On the one hand, not reporting the mean and SD and reporting different outcome measures made performing a meta-analysis impossible; on the other hand, a low sample size of studies, a vast variation in the duration of interventions, and a low number of eligible studies made it difficult to come to a definite conclusion about the effect of caffeine in PD. Moreover, they made data combining impossible.
5.2. Similarities and Differences in Relation to Other Studies
To the best of our knowledge, there is no published systematic review and meta-analysis about the effect of caffeine on PD symptoms. Previous studies evaluated the effect of caffeine on PD risk. Therefore, we could not compare our study with them.
5.3. Strengths and Weaknesses
This systematic review is the first to assess exclusively the clinical trials that evaluated the effect of caffeine supplementation on ESS and UPDRS in PD. All the included studies reported the side effects of caffeine. We included the clinical trials, to avoid the influence of the bias of observational studies. Well-designed RCTs are needed to be performed in the future.
Several limitations should be noted in our study. One of the limitations of this systematic review is the quality of trials included. Some of the trials were not well-blinded or properly randomized. The sample size of some included studies was small. In addition to the methodological limitations of the original studies, the duration of intervention was very different in different studies, which made it difficult to come to a definite conclusion about the effects of caffeine on PD. Finally, we assumed that most of the well-designed RCTs would have been published in English journals, and consequently, we restricted our search only to English studies.
5.4. Conclusion and Future Research
Although no effect of caffeine was reported in the studies, the lack of meta-analysis and the poor quality of some papers mean that there is insufficient evidence to make a judgment about the effectiveness of caffeine on PD. It is suggested that well-designed RCTs be conducted to compare the effect of caffeine with placebo for controlling the symptoms of PD. Further RCTs regarding the effects of caffeine with rigorous methods, larger sample sizes, adequate and well-reported allocation concealment, and blinding on the symptoms of PD would be helpful.
Finally, the authors should follow standard international guidelines such as CONSORT guidelines when reporting their results and should appropriately design the protocol before beginning a study.