1. Introduction
The global pandemic caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is omnipresent. The pathological cellular mechanisms that induce a cytokine release “storm” are believed to go through different phases, which lead to dysregulation of the immune system (1). As the angiotensin-converting-enzyme 2 (ACE2) receptor is a co-receptor for virus spike proteins, it might inflict indirect damage of the cerebrovascular endothelium cells due to an interaction of the viral “S” protein and the ACE2 receptor, leading to inflammation and apoptosis (2). Another mechanism of the pathophysiology of ICH in COVID-19 patients could be the direct interaction of the virus with the endothelium causing rupture of capillaries with subsequent intracerebral hemorrhage. The breakdown of the blood-brain barrier is also induced by pro-inflammatory cytokines, alterations in the coagulation cascade, and complement system. The risk of ICH could also be increased by an accumulation of angiotensin II in the local endothelium due to a downregulation of the ACE2 receptor by SARS-CoV-2, which then interacts with the renin-angiotensin-aldosterone system (3, 4). In this report, we present a rare case of ICH with delayed cerebral vasospasm (CV).
2. Case Presentation
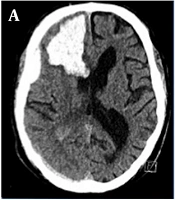
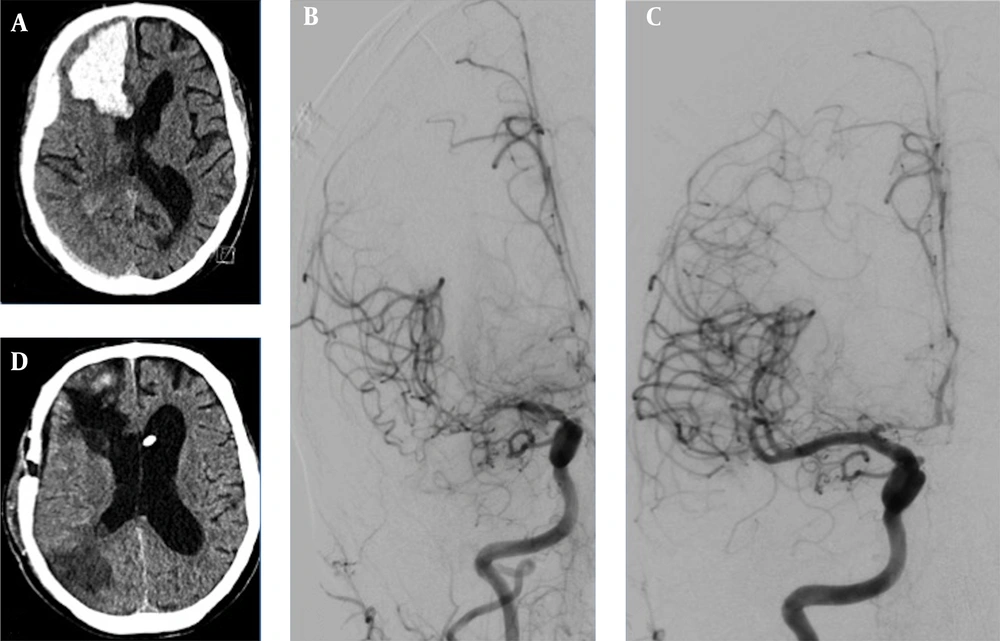
A 65-year-old male suddenly became unconscious while drinking alcoholic beverages. He celebrated a birthday with five families the day before, had a 39.5°C fever but did not follow the rules of social distancing. In his past medical history, he had a sick-sinus syndrome requiring a cardiac pacemaker and a 40 pack-year history. On the day of admission, the blood serum alcohol level was 4.3 g/L. He had no fever or neurological deficits, no cough, and only dyspnoea under physical load. Chest X-ray showed a density of the left basolateral lung without inflammatory reactions. SARS-Corona PCR (Cepheid and Hologic) was positive. D-Dimer was elevated with 9,696 ng/mL, inflammatory parameters were as follow: CRP 5.08 mg/dL, IL-6 95.1 pg/mL, and Thrombocyte count was 81/nL. Shortly after admission, he suffered from a generalized tonic-clonic seizure. CT-scan showed a space-occupying right frontal intracerebral hemorrhage (ICH) and concomitant subdural hematoma (Figure 1). CT-angiography (CT-A) and cerebral angiography (DSA) failed to show any vascular abnormalities. A high-resolution CT of the chest showed no features of COVID-19. Cranial CT follow-up revealed a progression of the ICH, which led to an immediate microsurgical evacuation. The postoperative period was unremarkable. Thirteen days following surgery, the patient became unconscious again. CT-A revealed severe cerebral vasospasm of the right MCA, both ACA and basilar artery requiring repetitive PTAs and intra-arterial nimodipine as well as oral nimodipine treatment regimes. After four days, vasospasm eventually resolved. Due to a malresorptive hydrocephalus, a ventriculoperiotoneal shunt was implanted on day 26 after the occurrence of the ICH. Final CT revealed infarction in the right posterior MCA territory (Figure 1). The patient was transferred to a rehabilitation center with a modified Rankin Scale score of 4.
A, initial cCT depicts right frontal ICH with subdural hematoma; B, in the course of hospital stay, cerebral vasospasm occurred on the 13th postoperative day (angiogram p.a.-projection of right ICA injection); C, after four times of PTA and local spasmolysis, vasospams of the ACA and MCA-territories resolved; D, final cCT after vp-shunt implantation revealed partial infarction of the right posterior MCA-territory.
3. Discussion
We report, to our knowledge, the first case of ICH with delayed severe cerebral vasospasms in a COVID-19. Neurovascular injuries after SARS-CoV-2 infection are one of the important manifestations, with a reported stroke incidence of 1 - 2% of all COVID-19 hospitalized patients (5). The relationship between spontaneous intracerebral hemorrhage and the SARS-CoV-2 infection remains unclear. A recent European multicenter study reported on 18 patients with acute intracranial hemorrhage. Although a direct association between the infection could not be proven, they concluded it might be more likely in patients with severe symptoms of COVID-19 (6). This is in line with previous reports that critically ill patients are prone to cerebrovascular complications (7). Our patient, however, had mild respiratory symptoms and was intubated due to his neurological deterioration. It is not clear whether a direct viral infection or an indirect pathomechanism such as an inflammatory reaction, dysfunction of the coagulation pathway, or an autoimmune mechanism causes cerebrovascular damage. There are at least three possible mechanisms responsible for the development of CV in COVID-19 patients. First, the ICH itself can induce CV due to CSF heme products that induce oxidative stress and inflammation (8, 9). Second, vasculitis is seen in cerebral vessels of patients with SARS-CoV-2, which can explain endothelial damage and vasoconstriction leading to CV (10). Third, a reversible vasoconstriction syndrome (RCVS) of cerebral arteries has been described (11, 12). Another patient succumbed to a devastating diffuse arterial and dural venous sinus construction with generalized cerebral edema four days after admission (13). It was hypothesized that SARS-CoV-2 might induce vasoconstriction due to downregulation of the ACE2 receptor, which then provoked an over activation of the renin-angiotensin-aldosterone axis. In non-COVID-19 patients, the RCVS can cause ICH in about 34 - 43% of cases, possibly due to a failure in cerebral autoregulation with reperfusion injury-causing rupture of cortical blood vessels (11, 14). In our patient, we suspect a strong association between the occurrence of the COVID-19, spontaneous ICH, and subsequent CV; however, the hypothesis of a direct interaction of the virus crossing the blood-brain barrier cannot be proven in our patient. The analysis of the CSF revealed no specific SARS-CoV-2-virus RNA in our patient. This finding does not exclude the suggested pathomechanism and is in accordance with other case reports (15).