1. Background
Surgical procedures induce a stress response (1), which increase reactive oxygen species (ROS) production and promote neurodegeneration (2). Endogenous antioxidants such as glutathione peroxidase (GPX), superoxide dismutase (SOD), and catalase (CAT) are meant to counteract ROS (3).
Dexmedetomidine (DEX), an α2 adrenoreceptor agonist with anxiolytic (4), sympatholytic (5, 6), cognitive advantages (7) and pulmonary-mechanics modifying effects (8), has been used to attenuate hemodynamic response during anesthesia (9) and surgery. It has also been used to alleviate stress response and ROS surge.
Spinal surgeries are of moderate stress level, which may propagate ROS (10). Thus, different techniques and medications might be used to attenuate oxidative response.
2. Methods
2.1. Anesthesia Method
In this randomized, blinded clinical trial, 56 patients (age range: 18 - 65 years) were included according to the classification of American Society of Anesthesiologists. The participants had no past medical history of spine surgery, body mass index (BMI) less than 30 kg.m-2, no addiction to opioids, alcohol, narcotics, or any illicit drugs, and they were candidates for single level lumbar spine laminectomy. Exclusion criteria were bleeding more than 10% of patient’s blood volume, duration of surgery more than four hours, change of surgical approach by the surgeon, and transfusion and inotrope administration during surgery. Four patients were excluded due to changes in surgical approach (decided by the surgeon during the procedure), and one patient was excluded following bleeding more than acceptable volume. Finally, 51 patients were included in the study.
Patients were allocated into two groups of Dex (D group; n = 25) and control (C group; n = 26). The surgery team and anesthesia technique were similar in both groups. Pulse oximetry (SpO2), non-invasive blood pressure monitoring (NIBP), capnometry (ETCO2), electrocardiogram (ECG), cerebral state monitoring (CSM), and train of four (TOF) were performed. Normal saline (5 mL/kg) was infused followed by midazolam (0.03 mg/kg) and fentanyl (3 mcg/kg) as premedication. General anesthesia was induced by injecting intravenous propofol (2 mg.kg-1), cisatracurium (0.15 mg.kg-1), and lidocaine (1 mg.kg-1). Moreover, propofol (100 - 300 mcg.kg-1.hr-1) was used to maintain cerebral state index (CSI) between 40 and 60.
Dex group received dexmedetomidine (0.6 µg.kg-1) as loading dose 15 minutes before induction of anesthesia, followed by 0.4µg.kg-1.hr-1 as maintenance. Control group received the same volume and sequence of normal saline. Trachea was intubated using properly sized endotracheal armored tube. During surgery, 50 µg of fentanyl was injected every 45 minutes until the end of surgery. If heart rate dropped to less than 40 beat per minute, atropine 0.5 mg was injected intravenously, and if mean arterial pressure was less than 60 mmHg, 10 mg of ephedrine was administered. In case of refractory hypotension, Dex infusion was decresed by 0.1 mcg.kg-1.hr-1, further followed by 20 mcg.kg-1.hr-1 decrease in propofol infusion rate. In case of rising mean pressure to more than 20% of baseline measures, trinitroglycerin was adminidtered (3 - 5 mcg.min-1). Anesthesiologist and surgeon were not informed about the groups and were blinded to the study. Dex discontinued after the skin closure at the end of surgery. During the process, heart rate and mean arterial pressure were documented every 15 minutes. Numeric Rating Scale (NRS) for postoperative pain was recorded just after admission to the post-anesthesia care unit (PACU). If NRS was greater than 3, a 2.5-mg bolus dose of morphine sulfate was administered and repeated according to further pain assessments. Also, Shivering Assessment Scale (BSAS) and post-operative nausea and vomiting (PONV) score on a 4-point verbal descriptive scale were utilized to evaluate shivering and nausea status of patients in the PACU (11, 12).
2.2. Biochemical Method
Venous blood samples (5 mL each) were collected right before anesthesia induction (T0) and immediately after entering PACU after surgery and emergence (T1). After collecting the sample, the serum was allowed to clot for 5 - 10 minutes at room temperature. Then, the sample was centrifuged at 500 - 1000 G-force for 20 minutes, and the supernatants were collected carefully. Next, the antioxidants activity levels were analyzed using Zellbio (GmbH, Germany) kits. GPX, SOD, and CAT enzyme activities were measured in both T0 and T1 according to instructions provided by the kit manufacturer (data S1, S2, and S3).
2.3. Randomization and Blinding
Randomization was done using a random number table generated by computer. Participants were randomly assigned into Dex or control groups by simple randomization procedures (computerized random numbers). Since the intervention applied after induction of anesthesia, patients were blinded to the study. In addition, the staff that processed the samples and measured the desired items were blinded to study groups and samples.
2.4. Statistical Method
Sample size was calculated based on previous studies (13-15) by considering the comparison means and using sample size calculation formula with G power application.
In this study, all quantitative variables were presented as mean ± SD, and the categorical variables were shown as percentage. Also, some ordinal variables were shown as median with IQR (inter quartile range) in different groups.
To compare continuous variables between the study groups, the Student’s t-test or non-parametric Mann-Whitney test was used alternatively. The categorical variables were compared using the Pearson chi-square test or Fisher’s exact test when appropriate.
To compare the longitudinal data (heart rate and mean arterial pressure) between groups, the mixed model analysis was performed.
All the statistical tests were two-tailed at the significance level of 5%, and SPSS 26 was used for data analysis.
2.5. Ethics
The Ethical Committee of Shahid Beheshti University of medical sciences, Tehran, Iran approved this study, and a clinical trial code was obtained IRCT20151012024493N4. Also, written informed consent was obtained from all participants. The procedures were in accordance with the Helsinki Declaration of 1975, as revised in 2000.
3. Results
The C and D patients were not different in terms of gender, age, BMI, and concomitant diseases (Table 1).
| Variables | Control Group | Dex Group | P-Value |
|---|---|---|---|
| Male/ female | 15 (57.7)/11 (42.3) | 15 (60.0)/10 (40.0) | 0.867 |
| Age (y) | 43.88 ± 12.78 | 48.68 ± 11.79 | 0.144 |
| BMI* (kg.m-2) | 27.47 ± 3.47 | 26.77 ± 2.13 | 0.102 |
| Concomitant diseases | 4 (15.4) | 0 (0.0) | 0.110 |
| Total | 26 | 25 | NA |
Demographic Characteristics of Participants
Although the serum levels of CAT and GPX increased during the procedure, these changes were not statistically significant (P-value o.579 and 0.762 respectively). SOD mean did not change over time in any of the groups. Also, SOD measures were not meaningfully different between the groups at any of the predetermined times (Table 2).
| Variables | Control Group | Dex Group | P-Value |
|---|---|---|---|
| CAT (U/mL) | |||
| Before | 2.57 ± 1.81 | 3.42 ± 3.61 | 0.935 |
| After | 4.89 ± 5.44 | 7.41 ± 14.14 | 0.607 |
| Diff. | 2.22 ± 6.33 | 3.98 ± 15.30 | 0.579 |
| GPX (U/mL) | |||
| Before | 170.22 ± 116.81 | 131.66 ± 102.28 | 0.260 |
| After | 258.68 ± 165.51 | 238.23 ± 170.41 | 0.786 |
| Diff. | 88.45 ± 198.19 | 106.57 ± 176.95 | 0.762 |
| SOD (U/mL) | |||
| Before | 82.49 ± 19.79 | 83.62 ± 15.24 | 0.860 |
| After | 84.24 ± 17.85 | 82.43 ± 27.60 | 0.567 |
| Diff. | 1.75 ± 30.26 | -1.19 ± 35.01 | 0.665 |
Serum Levels of Antioxidants Sampled Before Induction of Anesthesia and Immediately After Surgery in the Control and Dexmedetomidine Groups
Three clinical outcomes were statistically different between the groups: (1) propofol consumption; (2) postoperative pain scores; and (3) shivering scores (Table 3). The duration of surgery and the volume of blood loss were not meaningfully different between the groups.
| Variables | Control Group | Dex Group | P-Value |
|---|---|---|---|
| Duration of surgery (min) | 165.20 ± 34.73 | 178.20 ± 51.35 | 0.318 |
| Bleeding volume (mL) | 365.25 ± 133.88 | 444.80 ± 282.36 | 0.810 |
| Propofol consumption (mg) | 1715.80 ± 429.17 | 1351.60 ± 427.17 | 0.007 |
| NRS (0 - 10) | 5.80 ± 1.00 | 2.40 ± 0.96 | < 0.001 |
| PONV Score; median (IQR) | 0 (0 - 0.75) | 0 (0 - 0) | 0.242 |
| BSAS (0 - 3); median (IQR) | 1 (0 - 2) | 0 (0 - 1) | 0.030 |
Comparison of Clinical Outcomes Between the Control and Dexmedetomidine Groups
The amount of propofol consumed in DEX group was obviously less than control group, despite the similar surgery duration (1351.60 ± 427.17 mg in DEX group vs. 1715.80 ± 429.17 mg in control group, P-value = 0.007).
Pain scores measured in PACU were significantly less in DEX group (P-value < 0.001). The mean score reported for DEX group was 2.40 compared to 5.80 in the control group.
Based on Bedside Shivering Scale measures (0 - 3), the median score was 0 for DEX group and 1 for the control. This data was statistically interpreted in the form of interquartile range (IQR) and the difference was not significant (Table 3).
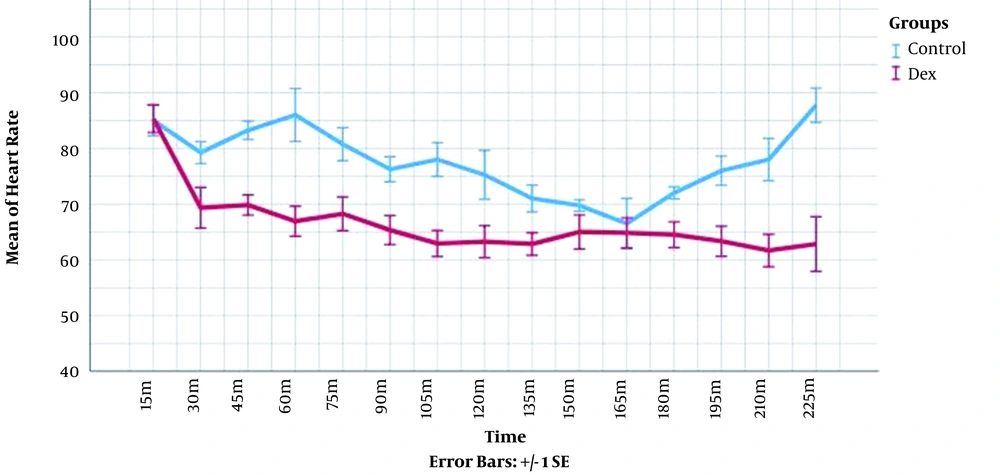
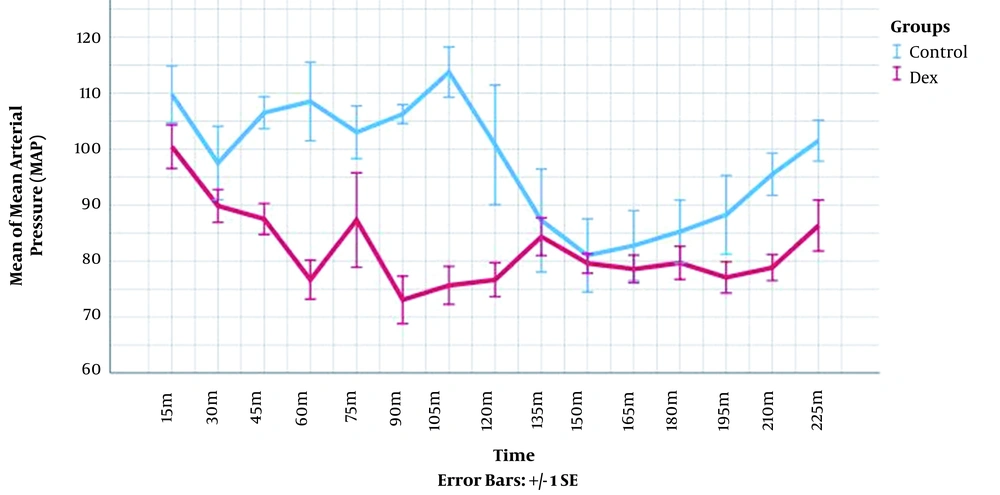
The heart rate and mean arterial pressure were measured in each time point for every patient and compared between the groups. The longitudinal changes over time were analyzed through mixed model analysis (Table 4). These measures were significantly lower in DEX group (P-value < 0.001). The visual demonstration of these data is presented in Figures 1 and 2.
| Variables | Control Group | Dex Group | P-Value a |
|---|---|---|---|
| Δ Heart rate | 78.41 ± 12.16 | 68.32 ± 11.23 | < 0.001 |
| Δ Mean arterial pressure | 96.53 ± 15.70 | 83.61 ± 14.13 | < 0.001 |
Mean Heart Rates (HR) and Mean Arterial Pressures (MAP) Changes over Time During the Surgery According to Mixed Model Analysis
4. Discussion
It has been demonstrated that different surgical procedures have strong impact on stress related responses (16). Other than surgery itself, several medical and non-medical processes could affect the inflammatory stress response, including various anesthesia medications and techniques (17-22).
On cellular level, exposure to volatile anesthetics manifested antioxidant effects on several cell types, except the neurons (23). Propofol has also proved to be a neuroprotective agent via its scavenging and immunomodulatory activities (24).
Desirable anti-inflammatory effects of DEX as a sedative in critical units are well known (25). In addition, perioperative adjunctive use of DEX in general anesthesia has been shown to considerably decrease serum IL6, IL8, and TNF-α level (26, 27). Furthermore, DEX blunts stress responses via several other pathways, such as attenuating norepinephrine (28), epinephrine (29), and cortisol (27) release during surgical procedures. One of several known pathways for anesthesia and surgery-related oxidative stress is via attenuating antioxidants, such as GPX, SOD, and CAT (30). In a recent study, in patients with mild to moderate concomitant brain injuries who underwent neurologic surgeries, DEX (1 µg.kg-1.hr-1) was infused for 15 minutes before tracheal intubation, which demonstrated to have neuroprotective, anti-inflammatory, and antioxidant effects. Plasma brain derived neurotrophic factor (BDNF), several cytokines, and SOD levels were indicators of the above properties, respectively. SOD levels were significantly higher in patients receiving DEX compared to control group (31). In 2015, Han et al. compared three groups of DEX, propofol, and midazolam; each group received the anesthetic as induction and maintenance agent (13). Venous blood samples were obtained prior to the surgery (T0) and 2 h (T1), and 24 h (T2) after the surgery. At T1, all three markers decreased, and then decreased to less than the baseline values at T2.
On the other hand, the hemodynamic effects of DEX are documented repeatedly, which is also true in our study. Shivering and pain controlling effects are also compatible with other studies. In our prior study in transsphenoidal resection of pituitary adenoma, DEX infusion improved surgeon’s satisfaction and decreased surgical site bleeding (32). However, current study did not indicate significant difference between groups regarding surgical blood loss.
SOD, GPX, and CAT play major roles as enzymatic antioxidant defense systems scavenging free oxygen radicals, mainly by reducing them into water. Inconsistent data about DEX effects on surgical stress responses have been reported. Various pathways have been assessed, and distinct surgical procedures may impact those responses or pathways differently. In the present study, particularly in laminectomy surgery, DEX did not affect the assessed antioxidants significantly. Further studies are recommended to clarify the possible mechanisms of observed discrepancies between various studies. Based on our findings, adjoining DEX to total intravenous anesthesia for simple spine surgeries may not affect antioxidant mechanisms significantly.
Equivocal findings in our study may be related to the limited number of cases. Therefore, larger studies with multiple measurements over an extended period of time could manifest more specific results. However, it should be noted that there are differences in the types of surgeries and their related stress responses. Laminectomy without dural puncture is a restricted source of oxidative stress compared to other neurosurgical procedures, and the results may vary substantially during other surgeries.
In conclusion, despite favorable hemodynamic and analgesic effects of dexmedetomidine, adjuvant infusion of this anesthetic during general anesthesia for laminectomy does not affect endogenous antioxidants during the surgery. Effects of the dexmedetomidine on antioxidant profile during postoperative period or in other spinal procedures are recommended for further studies.