1. Context
Vascular dementia (VaD) is the second most common type of dementia after Alzheimer’s disease (AD) worldwide. Vascular dementia is a neurodegenerative disorder characterized by gradual cognitive impairment. Ischemic and hemorrhagic strokes result in VaD, markedly disturbing cerebral blood flow and decreasing patients’ cognitive and memory performance (1-3). Neurons are more sensitive to hypoxic changes and mitochondrial stress than other cell types. Oxygen deprivation in neurons causes brain damage within minutes (4). The distribution of cerebral blood flow, which is the leading cause of VaD, affects patients’ cognitive and memory performance and declines energy metabolism (5). Mitochondrial dysfunction and selective autophagy of mitochondria, known as mitophagy, are associated with VaD. This review aims to elucidate the contribution of mitophagy to VaD.
2. Evidence Acquisition
This review was conducted independently by at least two researchers dominant in various types of VaD studies. We searched databases including Elsevier, Google Scholar, and PubMed using the terms ‘vascular dementia,’ ‘vascular cognitive impairment,’ and ‘mitophagy.’ We evaluated 70 articles on the relationship between VaD and mitophagy and interpreted the results. Adobe Photoshop 2022 was used for drawing figures by researchers.
3. Results
3.1. Role of Mitochondria in Vascular Dementia
Neurons are highly specialized and post-mitotic cells. They cannot eliminate degraded proteins or damaged organelles by dividing them into other cells by mitosis (6). Neurons require a large amount of adenosine triphosphate (ATP) to maintain neuronal activities, including anterograde and retrograde transports of neurotransmitters and membrane-bound organelles. Maintaining the resting membrane potential of neurons and restoring ionic balance after depolarization also occurs in the presence of ATP. To execute all these physiological processes, neuronal activities depend on mitochondrial function and oxygen supply more than other cell types (7-9). Mitochondria are the main organelles with prominent roles in energy production and regulation of calcium trafficking between intracellular and extracellular fluid (10). Mitochondrial maintenance is vital for neuronal development, function, and survival (11).
Several pathological mechanisms, including dysregulation of mitochondrial morphology, mitochondrial dysfunction, and autophagic cell death, are markedly observed in neurodegenerative disorders (12-14). It was stated that both autophagy and mitophagy had protective roles in various neurodegenerative diseases such as AD, Parkinson’s disease (PD), amyotrophic lateral sclerosis (ALS), Huntington’s disease (HD), and VaD by removing abnormally aggregated proteins and maintaining cell integrity via removing damage mitochondria (10, 15-17). Decreased energy metabolism and low nutrient availability in neurodegenerative diseases mainly damage the mitochondria (18, 19). Mitochondrial injury related to VaD has been demonstrated in previous studies (20-22). A decline in mitochondrial ATP and mitochondrial DNA (mtDNA) levels was observed in VaD-induced experimental rats (21, 23).
3.2. Neuroprotective Role of Mitophagy in Vascular Dementia
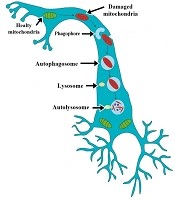
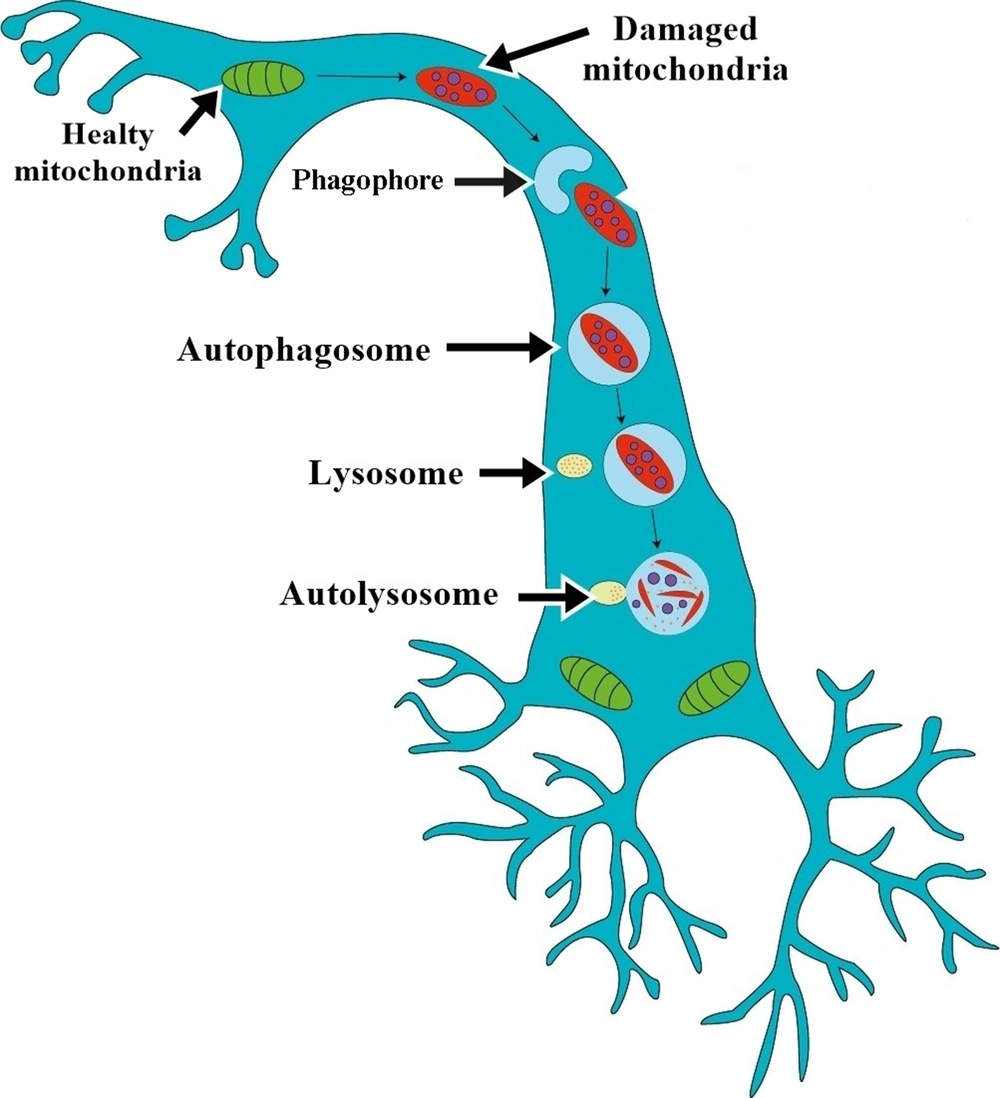
Autophagy is an intracellular degradation system that removes the aged or damaged organelles and unfolds or degraded proteins. It is characterized by forming and expanding an isolation membrane known as a phagophore (Figure 1). The phagophore fuses engulf cytoplasmic constituents in double-membrane structures known as autophagosomes (24-26). The autophagosomes deliver their substrates to lysosomes for degradation. Autophagosome fusing with lysosome is called autolysosome (27-29). This process is vital for balancing energy sources, survival during starvation, removing misfolded proteins, clearing damaged organelles, including mitochondria and endoplasmic reticulum, and cell differentiation (27).
Mitochondrial movement and mitophagy in neurons. Phagophore is known as an isolation membrane, surrounds damaged mitochondria, then fuses to mitochondria, newly formed double-membrane structures known as autophagosomes. The autophagosomes deliver mitochondria to lysosomes for degradation. Autophagosome fuse with lysosome is referred to as autolysosome.
Researchers have used ischemic rodent models, including chronic and focal hypoperfusion, subarachnoid hemorrhage, and cerebral ischemia-reperfusion, to understand vascular changes related to cognitive impairment (30-33). These models can be used to elucidate the pathophysiology of VaD. In previous studies, autophagy played a protective role in experimental VaD models via preserving vascular integrity and the structure of the blood-brain barrier, upregulating occludin and claudin protein expressions, reducing oxidative stress, and decreasing cognitive dysfunction (14, 22, 34, 35).
Selective autophagy of mitochondria, recognized as mitophagy, is an essential mechanism for removing aged and damaged mitochondria (15, 36). Eliminating damaged mitochondria by mitophagy is necessary for maintaining mitochondrial homeostasis and healing neuronal injury (37). Due to their high energy demands, neurons are more sensitive to changes in their cellular architecture (15, 38). It is increasingly recognized that removing misfolded and aggregated proteins, damaged DNA, and mitochondria are necessary for maintaining the normal function and the lifespan of neurons (39). Deposition of abnormal proteins, lipids, and clots in the brain tissue is detected in VaD, and those degraded molecules are removed by autophagy to prevent atypical vascular accumulations and maintain normal vascular biology (3).
Mitophagy levels vary in different regions of the brain. Mitophagy highly occurs in the dentate gyrus, lateral ventricle, and Purkinje cells, whereas mitophagy is rarely seen in the brain’s striatum, cortex, and substantia nigra region (39, 40). Age-related organelle damage increases neuronal mitophagy. Higher kinetics of neuronal mitophagy exacerbates development of neurodegenerative diseases (40-42).
Neurons have three compartments, including axons, dendrites, and soma, which differ from those of other cell types. Mitophagy may occur differently in neurons than in cells not containing cytoplasmic extensions. Neuronal mitochondria are mainly located in areas with high energy demand, including the distal parts of axons and dendrites, the nodes of Ranvier, presynaptic buttons, and postsynaptic densities away from the cell body (43-46). Unlike mitochondria, lysosomes are rarely found in axonal and dendritic extensions. The process of mitophagy may occur in cell bodies where the lysosomes are located (47-49). Mitophagosome undergoes maturation during its movement from the axon to the cell body. Due to the spatial limitations, rapid removal of damaged mitochondria is necessary for neuronal survival (39, 42). As discussed in the next section, the literature also noted that the phosphorylation of Miro by PTEN-induced putative kinase protein 1 (PINK1) accelerates rapid mitochondrial transport from axon to cell body (49).
Some studies claim that autophagy could have adverse effects in a time-dependent manner against neuronal injury (50, 51). Prolonged autophagy or overexpressed autophagic proteins induce ischemic injury and cause neuronal cells to undergo apoptotic cell death. Apoptotic cell death triggered by mitochondrial damage can cause cognitive impairment (52). Therefore, the elimination of damaged mitochondria is essential to minimize neuronal damage. Previous studies suggest that autophagy is rapidly activated and shows protective effects in the early stages of ischemia, but in the long period of ischemia, autophagy induces apoptotic cell death. The neuroprotective effect of autophagy is controversial; it is unclear whether autophagy has protective or damaging effects on VaD (10, 18, 19).
3.3. Mitophagy Pathways
3.3.1. PINK1/Parkin Pathway of Mitophagy in Vascular Dementia
Two major genes regulate mitophagy: first, mitochondrial kinase PINK1, and second, ubiquitin ligase Parkin. PINK1/Parkin-mediated mitophagy is a well-known mechanism for regulating mitochondrial dynamics and degradation of damaged mitochondria in response to stress (15, 36, 39, 53-55).
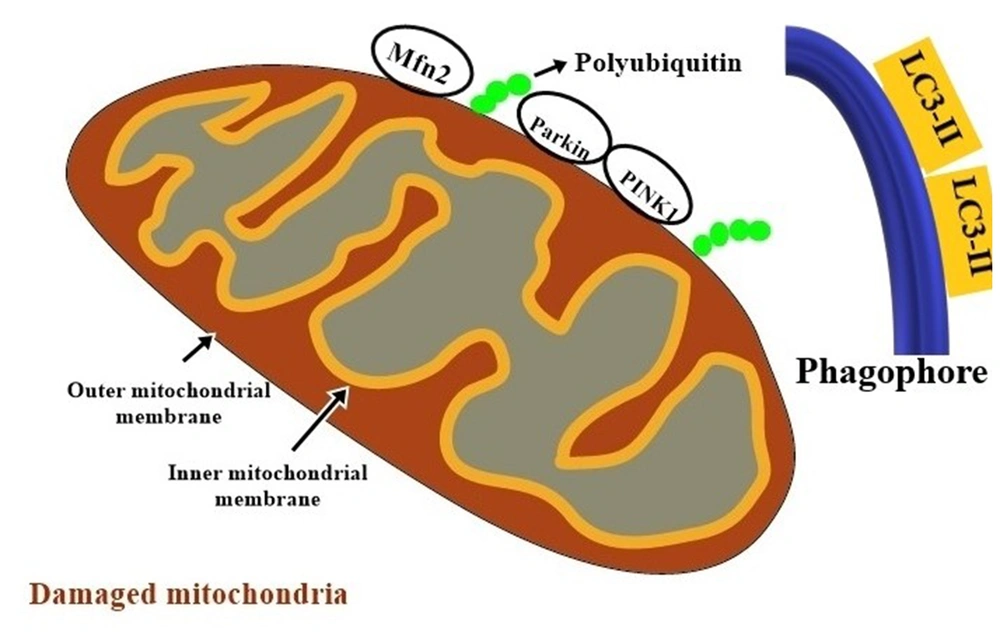
PINK1 is a serine/threonine kinase located in the inner membrane of mitochondria (56, 57). The roles of PINK1 and Parkin have been identified in both non-neuronal and neuronal cells of patients with hereditary PD. In response to mitochondrial damage and depolarization of its inner membrane, PINK1 accumulates on the outer mitochondrial membrane (OMM) (53, 57). Then, it induces phosphorylation of Parkin protein (Figure 2). Parkin bound to Mfn2 in a PINK1-dependent manner; PINK1 phosphorylated Mfn2 and promoted its Parkin-mediated ubiqitination, and mitophagosome formation. Production of microtubule-associated protein 1A/1B-light chain 3 II (LC3-II) promotes the maturation of phagophores.
PINK1/Parkin mediated mitophagy. In response to mitochondria damage and decreased potential of the mitochondrial inner membrane, putative kinase protein 1 (PINK1) accumulates on the outer mitochondrial membrane; then, it induces phosphorylation of Parkin protein. Parkin bound to Mfn2 in a PINK1-dependent manner; PINK1 phosphorylated Mfn2 and promoted its Parkin-mediated ubiqitination, and mitophagosome formation. Production of microtubule-associated protein 1A/1B-light chain 3 II (LC3-II) promotes the maturation of phagophores.
Parkin bound to mitofusin-2 (MFN2) allows for the induction of autophagy receptors and the formation of mitophagosome. Mitophagosomes fuse with lysosomes to degrade damaged mitochondria (25, 34). Parkin acts as a mitochondrial quality control center to maintain the organelle population by removing damaged mitochondria (55-61). The expression of PINK1 and Parkin was upregulated in VaD-induced rats (21). Miro is a Rho GTPase, primarily found in neurons and located on the outer mitochondrial membrane. It anchors mitochondria to microtubules, and the phosphorylation of Miro by PINK1 allows mitochondria to move from the axon to the cell body. This also protects neurons against mitochondrial stress.
Chronic cerebral hypoperfusion induces VaD, and activation of autophagy aggravates neuronal injury in brain tissue (12, 62). Microtubule-associated protein 1A/1B-light chain 3 II (LC3-II) is a crucial marker for autophagosomes and autolysosomes (63). Upregulation of LC3 protein and increased hippocampal LC3-II/ LC3-I levels were demonstrated in VaD in both in vivo and in vitro studies (21, 23). Decreased cerebral circulation increases mitochondrial dysfunction, triggers oxidative stress, and causes death in neurons and astrocytes (12).
3.3.2. Punctate-Mitochondria-Dependent Mitophagy Results
Mitochondrial fission is assumed to be crucial for mitophagy. Dynamin-related protein-1 (DRP1), a large GTPase, plays a major role in the mitophagic process. It is responsible for the selection of the damaged mitochondria for degradation.
DRP1 is also associated with four mitochondrial receptor proteins known as fission 1, mitochondria fission factor, and mitochondrial dynamics protein of 49 kDa and 51kDa (64, 65). The movement of DRP1 from the cytosol to mitochondria promotes fission, called punctate-mitochondria-dependent mitophagy. This process is crucial for mitophagy and plays a vital role in neurodegenerative disease (66). Expression of DRP1 in brain microvascular endothelial cell culture markedly increased after induced vascular damage. Punctate-mitochondria-dependent mitophagy in brain microvascular endothelial cells was obtained in vivo (67).
3.3.3. PI3K/Akt/mTOR Signaling Pathway Indirectly Induced Mitophagy
The phosphoinositide 3-kinase (PI3K)/protein kinase B (AKT)/mammalian target of the rapamycin (mTOR) pathway is crucial for the maintenance of cellular homeostasis via preventing abnormal cell division under physiological conditions. Overexpression of this pathway causes the initiation and progression of pathological diseases (68). It was shown that the inhibition of PI3K/Akt/mTOR pathways induced autophagy in middle carotid artery occlusion and hypoperfusion rat models (69, 70). In these models, it has been suggested that apoptosis of cells increases neuronal damage, and activation of autophagic pathways by suppressing apoptosis may prevent neuronal damage. Especially, enhancement of mitophagy by rapamycin possibly improve neuronal damage by inhibiting apoptosis in VaD-induced rats (21).
4. Conclusions
In conclusion, it was revealed that the autophagy process plays a protective role in VaD via preserving vascular integrity and the structure of the blood-brain barrier and reducing oxidative stress and cognitive dysfunction. Some studies claim that autophagy could have adverse effects in a time-dependent manner against neuronal injury. The pathogenesis of VaD is partially known; however, there is an urgent need to elucidate the mechanisms driving dementia pathogenesis. Although there are limited studies on the activation of mitophagy-related pathways in VaD, and the definitive role of mitophagy in neuronal healing is unclear, further research is needed to elucidate mitophagy pathways in neurons.