1. Background
Parkinson’s disease (PD) is a chronic, age-dependent neurodegenerative condition (1). The incidence of PD is increasing over time, and by 2030, the number of people with the disease will triple (2). There is evidence that the disease is present throughout the world, with studies in Africa producing hospital frequencies of 0.3 to 2.3% of neurological diseases (3).
Parkinson’s disease is a multifactorial disease, but the complex interplay between the various factors is only just beginning to be understood. Parkinson’s disease is primarily caused by aging (4, 5). Parkinson’s disease is also associated with environmental exposures and genetic susceptibility. Parkinson’s disease was associated with several environmental factors. Among these factors are pesticide exposure, head injuries, rural living, agricultural occupations, and drinking well water. Family history is associated with an increased risk of PD (6).
Despite the improvement of the Moroccan health system, there is limited epidemiological data on PD. The incidence and prevalence of this disease, however, increase with age. As life expectancy increases, projections indicate that the number of people over 60 will increase from 2.7 million in 2010 to 5.8 million in 2030 (7), possibly leading to an increase in the number of people with PD. According to a systematic analysis of epidemiological studies, PD prevalence increased by 39.7% in Morocco between 1990 and 2016 (8). Compared to other North African populations, Moroccans present a high genetic predisposition to PD due to the prevalence of mutations in the leucine-rich repeat kinase2 (LRRK2) and Parkine2 genes. These genes correlate with PD risk (9, 10).
2. Objectives
Research like this will provide a better understanding of the etiology of PD, primarily since genetic causes represent only 5 - 10% of cases, while 75% are of uncertain origin, known as idiopathic PD. The results of this study will serve as a basis for identifying at-risk groups in the population in order to develop new prevention and treatment strategies.
3. Methods
This study is a case-control study aimed at identifying various characteristics of individuals associated with PD risk. Researchers examined risk factors for the disease, identified individuals at risk, and compared their characteristics with those of healthy control subjects.
3.1. Participants and Criteria of Inclusion
In this study, 180 patients (males and females), who had previously been diagnosed with PD, were examined cross-sectionally. By the recommendation of the Ethics Committees of the Faculty of Medicine and Pharmacy of Marrakech, all selected patients gave their written consent. The control subjects were recruited from departments other than the Department of Neurology without a prior diagnosis of neurodegenerative disease. Each case was compared with two age-matched controls per year to minimize the possibility of confounding factors.
3.2. Size of Sample
Open-epi Calculator determines the Size. Under this formula, the minimum sample size is 540 participants, distributed over 180 cases and 360 controls, for a power (% chance of detection) of 80%.
3.3. Data Collection
The study participants were informed of the objectives and protocol of the study. A physician conducted a neurological and clinical examination following the consent in writing. Clinicians completed a detailed clinical questionnaire during an interview with the patient to collect data.
3.4. Variables of Interest
The socio-demographic data and other clinical information. Additionally, the log sheet included questions regarding occupational experience and duration of employment. There was a list of all occupations in which participants had been employed for an extended period. The Dictionary of Occupational Titles (DOT) was used to categorize jobs.
In this survey, lifestyle habits were asked about past or present habits. The subjects were also surveyed regarding the medical and surgical comorbidities associated with PD.
3.5. Statistical Analysis
SPSS version 21 was used to process and analyze the data. The percentages of numbers were compared between groups using both Student’s t-test and chi-square tests. The chi-square test was used for categorical variables and continuous variables. The Student’s t-test was used. Because of the inequalities in sample sizes, the equality of variances premise was systematically verified. The Welsch test was used if there was a significant difference. An adaptation of the Student’s t-test, the Welch t-test, is used for two samples with potentially unequal variances (11).
3.6. Analysis of Factors Associated with Parkinson’s Disease
The factors associated with the disease were analyzed using a conditional logistic regression model in which the variable to be explained was the presence or absence of disease. For the present study, that model was developed through the following steps: choosing the independent variables, exploring data and verifying associations (analysis of variable distribution), and conducting univariate and multivariate analyses. The odds ratio (OR) was used to quantify the strong association between the variables. A multivariate analysis was conducted using conditional logistic regression to estimate the adjusted odds ratio with confidence intervals. Variables inserted in a model were selected according to their clinical significance and statistical association with a dependent variable at a 20% significance level in the univariate analysis (P value of Wald’s test).
3.7. Modeling Strategy
In the analysis, all explanatory variables that appeared significant in the interpretation of a dependent variable were retained in a final model. The regression procedure is based on a stepwise approach.
3.8. Model Selection Criteria
In the end, all variables that were not significant on the 5% significant level were excluded from the model. Akaike Criterion was used to estimate the quality of the model. We used the Nagelkerk R2 to examine the model summary and verify its association strength.
3.9. Ethical Consideration
According to the Declaration of Helsinki and the requirements of the Medical Ethics Committee of the Faculty of Medicine and Pharmacy in Marrakech, the study group adhered to the ethical principles of biomedical research. The Ethics Committee also approved a protocol for the study of the Faculty of Medicine and Pharmacy of Marrakech.
4. Results
4.1. Comparison of Socio-demographic Variables Between Cases and Controls
Five hundred forty eligible participants (180 cases and 360 controls) were invited to participate in this study. Table 1 compares the general demographic characteristics of cases and controls. Cases and controls did not differ in age, with controls averaging 68.09 years (SD 11.3) (t = -0.278, P > 0.05). Due to the matching, the age distribution among controls was comparable. Males’ average age was 68.81 years (SD 10.84), and females’ average age was 67.67 years (SD 11.71). In total, 54.07% of the participants (n = 292) were males. There were significant gender differences between the case and control groups (P < 10 - 3). Cases and controls have similar education levels.
| Characteristics | Cases | Controls | P Value |
|---|---|---|---|
| Number of participants | 180 | 360 | |
| Age (y) | 68.09 ± 11.3 | 68.38 ± 11.24 | 0.78 b, c |
| Age (y) | 0.42 d | ||
| ≤ 50 | 13 (7.2) | 23 (6.4) | |
| > 50 | 167 (92.8) | 337 (93.6) | |
| Body mass index (kg/m2) | 25.3 ± 3.9 | 25.7 ± 3.2 | 0.2 b |
| Gender | |||
| Female | 63 (35) | 185 (51.4) | |
| Male | 117 (65) | 175 (48.6) | 0 .0001 d |
| Area of residence | 0.53 e | ||
| Urban | 108 (60) | 226 (62.7) | |
| Rural | 72 (40) | 134 (37.3) | |
| Level of education | 0.27 e | ||
| None | 92 (51.1) | 198 (55) | |
| Primary | 44 (24.4) | 92 (25.5) | |
| Secondary | 28 (15.6) | 53 (14.7) | |
| University | 16 (8.9) | 17 (4.8) |
a Values are expressed as mean ± SD or No. (%).
b Student’s t-test
c Age matching
d Fisher’s test
e Chi-square test
4.2. Comparison of Cases and Controls by Principal Occupation
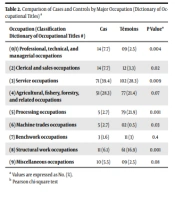
This study included a professional story. The dictionary of occupational titles (DOT) was used to code jobs. The DOT classified jobs into nine categories based on their similarity to other occupations. Both cases and controls in this study worked in the category of “service occupations” (cases 39.4%, controls 28.3%). Professionals in this group include military personnel, homemakers, housekeepers, janitors, and security guards. Table 2 shows that there was a significant difference between cases and controls by occupational and industrial category, except for benchwork occupations and agricultural, fishery, forestry, and related occupations (see Table 2). Comparisons were made between the patient and control groups. (Tables 1 and 2) present these comparisons.
| Occupation (Classification Dictionary of Occupational Titles #) | Cas | Témoins | P Value* |
|---|---|---|---|
| (0/1) Professional, technical, and managerial occupations | 14 (7.7) | 09 (2.5) | 0.004 |
| (2) Clerical and sales occupations | 14 (7.7) | 12 (3.3) | 0.02 |
| (3) Service occupations | 71 (39.4) | 102 (28.3) | 0.009 |
| (4) Agricultural, fishery, forestry, and related occupations | 51 (28.3) | 77 (21.4) | 0.07 |
| (5) Processing occupations | 5 (2.7) | 79 (21.9) | 0.001 |
| (6) Machine trades occupations | 5 (2.7) | 02 (0.5) | 0.03 |
| (7) Benchwork occupations | 3 (1.6) | 11 (3) | 0.4 |
| (8) Structural work occupations | 11 (6.1) | 61 (16.9) | 0.001 |
| (9) Miscellaneous occupations | 10 (5.5) | 09 (2.5) | 0.08 |
a Values are expressed as No. (%).
b Pearson chi-square test
4.3. Univariate Analysis
The univariate Logistic regression analysis revealed that the distribution of gender among both populations was different: there was a preponderance of males in cases. There was a 1.96-fold (CI 95% 1.35 - 2.84) increase in the risk of the disease in males compared to females. Cases (n = 43, 23.9%) had more family history of PD as controls (n = 18, 5%). Family history of PD correlated with significantly increased risks of PD (OR = 5.96, (CI 95% 3.32 - 10.70)). Also, a previous head injury is a risk factor with an OR of 3.41 (CI 95% 1.51-7.68). Additionally, a history of diabetes is associated with an Odds Ratio of 1.12 (CI 95% 0.76 - 1.66) but is not statistically significant. The use of medications for a condition other than PD was less frequent in the group with PD than in the controls. The use of these medications was associated with a decreased risk of PD (OR = 0.55, (CI 95% 0.38 - 0.79)).
The risk of the disease is increased with lower levels of education. Compared with university-level instruction, people without any level of schooling have an odds ratio of 2.02 (CI 95% 0.98 - 4.78). The consumption of well - water constitutes a PD risk factor with odds ratios of OR = 1.93, (CI 95% 1.34 - 2.78). On the contrary, source drinking was significantly and negatively associated with the risk of disease (OR = 0.29, (CI 95% 0.20 - 0.43)). Bivariate analysis with definite smoking status in three classes indicated an inverse association between tobacco use and disease risk (OR = 0.69, (CI 95% 0.26 - 1.79)), but not significantly compared to non - smokers. We observed a stronger association for former smokers (OR = 1.65, (CI 95% 1.04 - 2.64)). A significant inverse association was reported with coffee consumption (OR = 0.46, 95% CI 0.32 - 0.66). Such association was mainly detected for consumption of more than 21 years (OR = 0.3, CI 95% 0.12 - 0.71) (Table 3).
| Cases (n = 180) a | Controls (n = 360) a | Univariate Analysis (OR (IC à 95%)) | Seuil P | |
|---|---|---|---|---|
| Gender | ||||
| Female | 63 (35) | 185 (51.4) | Reference | |
| Male | 117 (65) | 175 (48.6) | 1.96 (1.357 - 2.841) | 0.0001 |
| Origin | ||||
| Urban | 108 (60) | 226 (62.8) | Reference | ------ |
| Rural | 72 (40) | 134 (37.2) | 1.12 (0.77 - 1.62) | 0.5 |
| Level of education | ||||
| None | 92 (51.1) | 198 (55) | 2.02 (0.980 - 4.781) | 0.05 |
| Primary | 44 (24.4) | 92 (25.5) | 1.96 (0.910 - 4.256) | 0.08 |
| Secondary | 28 (15.5) | 53 (14.7) | 1.78 (0.783 - 4.053) | 0.16 |
| University | 16 (9) | 17 (4.8) | Reference | ------ |
| Body mass index (kg/m2) | ||||
| < 18.5 | 1 (0.6) | 2 (0.6) | Référence | ------ |
| 18.5 au 24.99 | 87 (48.3) | 148 (41.1) | 1.34 (0.93 - 1.92) | 0.11 |
| ≥ 25 à < 30 | 80 (44.4) | 176 (48.9) | 0.83 (0.58 - 1.19) | 0.32 |
| ≥ 30 | 12 (6.7) | 34 (9.4) | 0.68 (0.34 - 1.35) | 0.27 |
| Residence near an agricultural area | ||||
| Yes | 59 (32.7) | 93 (25.8) | 1.39 (0.94 - 2.06) | 0.09 |
| No | 121 (67.3) | 267 (74.2) | Reference | ------ |
| Water consumption | ||||
| Well water | ||||
| Yes | 104 (57.8) | 149 (41.4) | 1.93 (1.34 - 2.78) | 0.001 |
| Never | 76 (42.2) | 211 (58.6) | Référence | ------ |
| Spring water | ||||
| Yes | 54 (33.9) | 218 (60.6) | 0.29 (0.20 - 0.43) | 0.001 |
| Never | 126 (66.1) | 142 (39.4) | Reference | ------- |
| Use of pesticides | ||||
| Yes | 26 (14.4) | 36 (85.6) | 1.51 (0.88 - 2.60) | 0.12 |
| No | 154 (42.7) | 324 (57.3) | Reference | ------ |
| Family history of PD | ||||
| Positive | 43 (23.9) | 18 (5) | 5.96 (3.32 - 10.70) | 0.001 |
| Negative | 137 (76.1) | 342 (95) | Référence | ------ |
| Use of drugs | ||||
| Yes | 69 (38.3) | 191 (53) | 0.55 (0.38 - 0.79) | 0.001 |
| No | 111 (61.7) | 169 (47) | Reference | ------- |
| Medical and surgical history | ||||
| History of head injury | ||||
| Yes | 16 (8.9) | 10 (2.8) | 3.41 (1.51 - 7.68) | 0.003 |
| No | 164 (91.1) | 350 (97.2) | Reference | -------- |
| High blood pressure | ||||
| Yes | 47 (26.1) | 96 (26.7) | 0.97 (0.64 - 1.45) | 0.89 |
| No | 133 (73.9) | 264 (73.3) | Reference | ------- |
| Diabetes | ||||
| Yes | 55 (30.5) | 101 (28) | 1.12 (0.76 - 1.66) | 0.54 |
| No | 16 (69.5) | 8 (72) | Reference | ------ |
| Operation under general anesthesia | ||||
| Yes | 31 (17.2) | 96 (26.6) | 0.57 (0.36 - 0.89) | 0.01 |
| No | 147 (82.8) | 263 (73.4) | Reference | ------ |
| Lifestyle | ||||
| Smoking status | ||||
| Never | 136 (75.5) | 239 (66.4) | Reference | ------ |
| Smoker | 6 (3.4) | 17 (4.7) | 0.69 (0.26 - 1.79) | 0.45 |
| Ex-smoker | 38 (21.1) | 50 (28.9) | 1.65 (1.04 - 2.64) | 0.03 |
| Coffee consumption | ||||
| Never | 99 (55) | 130 (36.1) | Reference | ------ |
| Yes | 81 (45) | 229 (63.9) | 0.46 (0.32 - 0.66) | 0.0001 |
| Duration of coffee consumption (y) | ||||
| Never | 99 (55) | 130 (36.1) | Reference | ------ |
| 1 - 10 | 35 (19.4) | 95 (26.4) | 0.27 (0.16 - 0.47) | 0.001 |
| 11 - 20 | 12 (6.7) | 17 (4.7) | 0.57 (0.31 - 1.04) | 0.07 |
| 21 - 30 | 12 (6.7) | 13 (3.6) | 0.3 (0.12 - 0.71) | 0.007 |
| > 30 | 22 (12.7) | 104 (29.2) | 0.22 (0.09 - 0.56) | 0.001 |
a Values are expressed as No. (%).
There were also higher odds ratios for technical and management occupations (OR = 4.25, (CI 95% 1.68 - 10.73)), clerical and sales occupations (OR = 2.95; (CI 95% 1.28 - 6.78)) and service occupations (OR = 1.53, (CI 95% 1.05 - 2.24)) in the case/control occupations analysis. Processors were found to have a lower risk of PD (OR = 0.07; (CI 95% 0.02 - 0.22). The protective associations ranged from 0.07; (CI 95% 0.02 - 0.22) for professionals in processing occupations to 0.31; (CI 95% 0.16 - 0.61) for those in structural occupations.
The risk estimation was not statistically significant for agriculture - related activities and metalworking occupations. OR = 1.22; (CI 95% 0.79 - 1.88) and OR = 1.08; (CI 95% 0.423 - 2.765), respectively. In contrast, homemakers had a high risk of contracting the disease (OR = 1.74, CI 95% 1.11 - 2.72). Previous work on a farm significantly reduced the risk (OR = 0.42; (CI 95% 0.27 - 0.63)). In Table 4, we present the odds ratios associated with specific occupations among both groups.
| Occupation | Cases a | Controls a | OR, CI 95% | P |
|---|---|---|---|---|
| Occupation (classification DOT) | ||||
| Occupation (classification DOT) | ||||
| (0/1) Professional, Technical, and Managerial Occupations | 14 (7.8) | 07 (1.9) | 4.25, 1.68 - 10.73 | 0.002 b |
| (2) Clerical and Sales Occupations | 14 (7.8) | 10 (2.8) | 2.95, 1.28 - 6.78 | 0.01 b |
| (3) Service Occupations | 68 (37.8) | 102 (28.3) | 1.53, 1.05 - 2.24 | 0.02 b |
| (4) Agricultural, Fishery, Forestry, and Related Occupations | 51 (28.3) | 77 (21.4) | 1.45, 0.96 - 2.19 | 0.07 |
| (5) Processing Occupations | 4 (2.3) | 80 (22.2) | 0.07, 0.02 - 0.22 | 0.001 b |
| (6) Machine Trades Occupations | 5 (2.8) | 02 (0.5) | 5.11, 0.98 - 26.62 | 0.05 |
| (7) Benchwork Occupations | 3 (1.7) | 11 (3) | 0.53, 0.148 - 1.95 | 0.3 |
| (8) Structural Work Occupations | 11 (6.1) | 62 (17.2) | 0.31, 0.16 - 0.61 | 0.001 b |
| (9) Miscellaneous OccupationsOccupation | 10 (5.4) | 09 (2.7) | Reference | |
| Farmer and related activities | ||||
| Yes | 41 (22.7) | 70 (19.4) | 1.22, 0.79 - 1.88 | 0.9 |
| No | 139 (77.3) | 290 (80.6) | Reference | |
| Housewife | ||||
| Yes | 43 (23.9) | 55 (15.3) | 1.74, 1.11 - 2.72 | 0.01 b |
| No | 137 (76.1) | 305 (84.7) | Reference | |
| Metalworking | ||||
| Yes | 07 (3.9) | 13 (3.6) | 1.08, 0.42 - 2.75 | 0.87 |
| No | 173 (96.1) | 347 (96.4) | Reference | |
| Previous work on a farm | ||||
| Yes | 45 (25) | 115 (31.9) | 0.42, 0.27 - 0.63 | 0.001 b |
| No | 135 (75) | 145 (68.1) | Reference |
Abbreviation: DOT, major divisions of dictionary of occupational titles.
a Values are expressed as No. (%).
b Significant at 0.05.
4.4. Multivariate Analysis
In the multivariate analysis, we used a descending stepwise logistic model with Wald’s test probabilities of no more than 20%. With the addition of the area of residence (forced factor), we included only the 16 variables significantly associated with PD risk in univariate analysis. The logistic regression model considered all variables for which the P value was less than 0.05.
The interaction effects between the variables were detected. A final model is composed of the nine non-interactive variables at the end of the step-by-step descending procedure. Multivariate analysis revealed significant associations of several predictors with PD in this population when independent variables were introduced descendingly. Spring water consumption exposes a person to a risk of 3. In contrast, familial PD is associated with a positive association. There is a seven-fold increase in disease risk. In this study, the use of drugs contributed to the risk for PD (ORa = 2.12, (CI 95% 1.33 - 3.38)), whereas surgery under general anesthesia was significantly negatively associated with PD (ORa = 0.47, (CI 95% 0.27 - 0.83)). There was a 3-point (P × 0.05) higher risk of PD for non-coffee drinkers after adjusting for age and smoking. The results indicate a significant inverse association between past farm work and depression (ORa = 0.30 with a 95% confidence interval (CI 95% 0.16 - 0.54)). It appears that working in processing occupations ORa = 0.05, (CI 95% 0.01 - 0.18) is protective of the occurrence of the disease. Based on the baseline data, the coefficient of adjustment measures the fit of the model. Cox & Snell have an R-square of 33%, Nagelkerke has an R2 of 48%, and McFadden has an R2 of 30% for this study. Those values indicate a positive correlation between the dependent and independent variables. According to this model, 48% of the variance of the studied variable can be explained by this model.
5. Discussion
Multifactorial pathogenesis of PD requires the identification of the population at risk. Many epidemiological studies (12) support a hypothesis of an increased risk of development of PD in the male population. Our findings are consistent with those of these earlier investigations. In the present research, when the gender factor was separately analyzed or adjusted (by age and area of residence), a significantly enhanced risk of the disease was observed for the males (crude OR = 1.96, (CI 95% 1.35 - 2.84), and (ORa= 1.92, (CI 95% 1.16 - 3.16)), both respectively.
Several studies have also reported an association between education and PD. The lowest level of education in this study was associated with a positive risk (crude OR = 2.02, (CI 95% 0.980 - 4.781)). The results of (13), therefore, are confirmed.
There is a link between PD and rural living, well and spring water use, and pesticide exposure (14). It examined the exposure to environmental factors, including the location of residence, the source of drinking water, and the exposure to pesticides and various chemicals at home and work. Several environmental factors related to the disease have been linked to the place of residence in previous studies (15, 16). The unadjusted analyses, independently of the other factors, disclosed no significant association between residential exposures (crude OR = 1.49, (CI 95% 1.00 - 2.20)) and PD. It is consistent with Hancock et al. (2008), who reported that residence or working on a farm was not significantly associated with an increased risk of PD (17). While matching was only done according to age, that result may be explained by the previous population immigrating to the urban region. Several epidemiological studies have linked well water to an increased risk of PD (18, 19), but this has not been confirmed in other populations (20-22). According to the findings of this study, drinking well water is associated with the disease in univariate, unadjusted analysis (crude OR = 1.93, (CI 95% 1.34 - 2.78)). It is suspected that healthy water may act as a vector for toxicants, possibly causing PD. Studies have also found positive univariate associations between source drinking water use and PD (23). Spring water use was negatively associated with PD in the current study (crude OR = 0.29, (CI 95% 0.20 - 0.43)). In stratified analysis, the consumption of well-water was excluded from the multivariate analysis as a risk factor unrelated to PD. Healthy water consumption probably interacted with rural residency and pesticide exposure to create confusion. The association between spring water use and PD persisted in multivariate analysis, with an ORa of 3.31 (CI 95% 2.05 - 5.34) in favor of a positive correlation. Although healthy and spring water composition may differ, the reason for the difference was unclear. Several studies have found that working in agriculture increases the risk of PD (18, 24). In univariate conditional logistic regression analysis, work in agriculture and agriculture-related activities was associated with more significant risks (raw OR = 1.22, (CI 95% 0.79 - 1.88)) but not significantly. The same results have been reported in other studies (13). After adjusting for other predictors (e.g., pesticide use, well water consumption, living area), the risk associated with these activities disappeared in the multivariate analysis. In order to further explore the question of work-related exposures as a contributing factor to the development of diseases in the population under study, we examined both agricultural activities and occupations involving pesticides and other chemicals.
It has been reported that pesticides are primarily responsible for PD development (13, 25). Numerous epidemiological studies have shown that pesticide exposure at work is associated with the disease. Pesticide use was associated with an increased risk (crude OR = 1.51, (CI 95% 0.88 - 2.60)), but not significantly. That study did not cover some information on pesticide use (use of equipment, protection, duration, and type of pesticide. Because of the relatively lower prevalence of pesticide manipulators in this study, we were also unable to establish the association of pesticides with PD, as reported earlier in a Washington study (26). Consequently, it may help investigate additional occupational exposures associated with PD, such as endotoxin (lipopolysaccharide) bacteria, common in various occupational and environmental settings, including agriculture (27, 28).
In the environmental risk factors, long-term experiences of living, such as occupation, can be critical. In the present study, consideration has been given to elucidating other occupational exposures while also considering the major confounding factors (cigarette smoking). The occupational activities employed during the most extended period were coded using the DOT and stratified into nine major groups. It showed that high crude risk was associated with working in agriculture, fishing, forestry, and related occupations, but this result was not statistically significant. An earlier study (29) confirms this observation. There may be an explanation for this by the fact that a large proportion of the study population is located primarily in urban and peri-urban areas that have little agriculture, which may have the effect of masking the association. Based on the results of this study, increased risks were observed for the occupation category of “clerical and sales occupations” and for the occupation category of “service occupations”. According to an unadjusted analysis, the personnel employed in service occupations (i.e., military officers, officials, medical and nursing professionals, and homemakers) were 1.53 times more likely to develop this disease than those employed in other occupations. It is only partially consistent with the results of the literature (26). There may be an explanation for such an outcome in that PD was recognized and acknowledged earlier in the educational and healthcare sectors (30).
To examine any confounding or interaction among the exposure variables, a multivariate analysis (Table 5) was conducted, followed by a logistic regression procedure. As a result of adjusting for potential confounders (gender, smoking), the results indicate that there is a decreased risk of PD in processing occupations (aluminum, mining, and ironworkers), which is consistent with several other studies (26), and not consistent with other studies that have shown an increased risk of the disease. Despite the difficulty in interpreting these results, classifications using the DOT cover a wide range of work activities and exposure potentials.
| Factors Selected | Beta | AIC | Error | aOR a | CI 95% a | Seuil P |
|---|---|---|---|---|---|---|
| Previous work on a farm | -1.19 | 517.63 | 0.33 | 0.30 | 0.16-0.54 | 0.003 |
| Use of drugs | 0.75 | 510.92 | 0. 23 | 2.12 | 1.33-3.38 | 0.02 |
| History of head injury | 1.21 | 505.73 | 0.54 | 3.38 | 1.16-9.83 | 0.02 |
| No consumption of coffee | 1.11 | 521.67 | 0.34 | 3.04 | 1.56-5.90 | 0.001 |
| Processing occupations | -2.85 | 562.59 | 0.85 | 0.05 | 0.01-0.18 | 0.001 |
| Operation under general anesthesia | -0.74 | 507.79 | 0.28 | 0.47 | 0.27-0.83 | 0.009 |
| Consumption of spring water | 1.19 | 525.92 | 0.23 | 3.31 | 2.05-5.34 | 0.001 |
| The notion of familial Parkinson’s | 1.97 | 530.86 | 0.36 | 7.19 | 3.41-15.13 | 0.001 |
| Male gender | 0.65 | 507.27 | 0.25 | 1.92 | 1.16-3.16 | 0.01 |
| Constant | -2.23 | |||||
Abbreviations: aOR, adjusted odds-ratio; CI, confidence interval; AIC, Akaike information criterion
a Calculated by logistic regression analysis for the age difference ~ 5 years between cases and controls
Medical and tobacco smoking histories, as well as information regarding family history, were also examined in this case-control study. Numerous epidemiological studies have demonstrated that individuals with a history of head injuries have an increased risk of developing PD (31-33). Additionally, we found a significant association between individuals with a history of head injury and the disease (crude OR = 3.41, (CI 95% 1.51 - 7.68)). Consistency of the evidence from multiple studies and different populations supports the conclusion that previous head injury is an etiological factor in PD.
Based on this study, diabetes and high blood pressure do not increase the risk of PD; crude OR = 0.97, (CI 95% 0.64 - 1.45) for diabetes and crude OR = 0.91, (CI 95% 0.61 - 1.34) for high blood pressure. Other studies have found an inverse association between hypertension and diabetes (34); however, other studies have found a positive association between diabetes and hypertension (35, 36). Differences in the population studied and the protocol used may explain these variances. In both univariate and multivariate analyses, drug use (crude OR = 0.55, CI 95% 0.38 - 0.79) and general anaesthesia history (crude OR = 0.57, CI 95% 0.36 - 0.89) were significantly associated with PD. Medication use was a significant risk factor in the logistic regression model with other PD risk factors (ORa = 2.12, (CI 95% 1.33 - 3.38)). Parkinson’s disease is well documented to have a genetic etiology (21, 37). Parkinson’s disease is more likely to occur in people with a family history. An unadjusted odds ratio of 5.96 was observed in this study, with a 95% confidence interval of 3.32 - 10.70. Statistically significant associations with PD persisted in the multivariate analysis (ORa = 7.19 (CI 95% 3.41 - 15.13)), suggesting that a positive family history of PD is an independent risk factor. Therefore, genetic factors may also be involved. Personal lifestyle habits, such as smoking and drinking, have been studied concerning PD risk. Based on the univariate analysis, coffee consumption was found to have a low protective effect against PD (crude OR = 0.46, (CI 95% 0.32 - 0.66)), most notably over 30 years (crude OR = 0.22, (CI 95% 0.09 - 0.56)). Accordingly, the results are consistent with the results of previous studies (14, 38-40), which indicated that coffee consumption is a negative predictor of PD risk.
In a stratified analysis controlling for other potential risk factors for PD, such as family history, history of severe head injury, area of residence, and age, non-consumption of coffee was found to be a significant risk factor for PD development (ORa = 3.04, (CI 95% 1.56 - 5.90)). The results of the present study support some hypotheses about how coffee drinking may benefit PD. Dopaminergic neurons of substantia nigra are protected from excitotoxic lesions by caffeine’s adenosine 2A receptor antagonist (41).
A final model (Table 5) identified family PD as the most significant predictor of PD risk, followed by previous head injuries and later spring water consumption. In the adjusted multivariate analysis, medication use was a significant risk factor for PD. The odds of contracting the disease were higher for men (ORa = 1.92, CI 95% 1.16 - 3.16) than for women. The risk of having disease following surgery with general anesthesia, as well as among those in the treatment professions, appeared to be reduced after adjustment for other risk factors. In our study, previous work on a farm reduced PD risk by 30%.
5.1. Conclusions
A serious public health issue of PD in Morocco has significant health, social, and economic repercussions.
The final PD model of risk suggested that the environmental and possibly genetic factors played an essential role in the etiology of the disease in this series. According to the present case-control study, positive family history is the primary nonenvironmental risk factor for PD, indicating the importance of expanding understanding of the genetic underpinnings of this disease. There is also an association between drinking spring water and PD. In the study sample, the male gender was also confirmed as a factor associated with the disease and had high statistical significance. However, considered classical risk factors for the disease, agricultural work, healthy water consumption, and residence in rural areas were not significantly different between cases and controls.
A single risk factor, therefore, is likely to pose a low risk and will likely differ depending on the specific characteristics of the population. Future research will need to identify additional risk factors.