1. Background
Road accidents and related mortality are one of the challenges of human society, which threaten public health and impose enormous costs on governments (1, 2). The rate of injuries and the frequency of major road accidents are so great that it is called road warfare (1). The traffic experts have identified the factors affecting the incidence and severity of crashes, including human-related factors, road-related factors, and vehicles (3). Analysis of road accidents in Iran showed that the human-related factor contributed to 97.5% of all accidents, followed by the environmental factor and vehicle with the rate of 70.5% and 31.5%, respectively (3). According to international traffic definitions, human-related factors, which are one of the main causes of traffic accidents in Iran (4), include driver’s mental health, personality traits, or psychiatric disorders (5).
Driving is a complex behavior pattern influenced by conscious and unconscious factors called "cognitive-behavioral characteristics" (1).
Depression is one of the most frequent psychiatric disorders affecting driving performance, which causes significant consequences (6). Cognitive impairment and psychomotor retardation, which are common reported symptoms of depression or the side effects of psychotropic drugs, can impair driving as an important social activity (7-9).
Several specific skills are essential for driving. As drivers have limited time to make the right decision and response, alertness, visual perception, selective attention, reactivity, and stress tolerance are of great significance (10). Many of these cognitive abilities may be affected in patients with depressive disorder. Most road accident studies have focused on socio-demographic characteristics and general personality as well as driving skills (11). While few studies have addressed driving performance considering depression and anxiety, their main purpose has often been the effects of medications, specifically antidepressants, and benzodiazepines, on road accidents (12-14).
2. Objectives
No study has been conducted on driving characteristics with a specific reference to patients with mental disorders (15). Therefore, the aim of this study was to examine whether depression affects driving performance. Hopefully, by identifying the factors, which may affect driving skills, traffic accidents and the resulting deaths will be reduced.
3. Methods
3.1. Participants and Design
This cross-sectional study was done to compare driving performance in depressed patients and a group of healthy controls. The subjects were selected from patients who were referred to outpatient and inpatient psychiatric units affiliated with the Shiraz University of Medical Sciences in 2021. Sampling was done by available and non-random methods. A sample of 100 participants was enrolled in this study who were divided into two groups, depressed patients and the healthy control group, according to psychiatric interviews. According to the previous studies and the normality of the data distribution, 50 people were placed in each group, considering the inclusion and exclusion criteria.
All participants signed informed consent. The researcher interviewed each participant for 40 minutes to diagnose any neurological or psychiatric illness. The patients previously diagnosed with major depressive disorder (MDD) (considering DSM5 criteria) were no exception and were re-interviewed to rule out other psychiatric illnesses, such as bipolar or psychotic disorders.
All the participants were between 18 and 60 years old, had at least a sixth-grade education, possessed a valid driving license, and were regularly driving during the last year.
The exclusion criteria were as follows: (1) having a neurological disease; (2) having a debilitating psychiatric disorder other than MDD; (3) having a comorbid substance use disorder; (4) receiving electroconvulsive therapy in the last six months; (5) not regularly driving during last year.
At first, all the participants were fully explained how to complete the questionnaires and do computer-based tasks. After making sure that they were understood, they were asked to complete the following questionnaires, and the computer test started.
3.2. Demographic Questionnaire
A demographic questionnaire was used to document information about age, gender, level of education, occupation, marital status, type of driver’s license, and history of collisions (irrespective of responsibility) involved as a driver in the last year.
3.3. Manchester Driving Behavior Questionnaire
This scale was developed and arranged by Reisen et al. in the Psychology Department of Manchester University, UK. It is based upon the idea that errors and violations have different psychological causes and methods of correction. Therefore, they must be differentiated by researchers. Manchester Driving Behavior Questionnaire (MDBQ) has become a popular tool for assessing driving behaviors. This scale has 50 questions scored on a Likert scale ranging from zero to five. The questions have two different aspects. One aspect is related to the type of behavior and the second aspect is related to the amount of danger that is created for other drivers. Abnormal behaviors include anomalous errors, slips, and intentional and unintentional violations. These behaviors are classified as follow: (1) behaviors that pose no danger to others and only make them feel comfortable (low risk); (2) behaviors that are likely to endanger others (medium risk); (3) behaviors that are sure to put others at risk (high probability).
MDBQ has acceptable psychometric properties. Parker et al. (16) obtained correlation coefficients of 0.81 for errors and 0.75 for reliability violations by researching 80 drivers seven weeks apart. Moreover, Oreizi and Haghayegh reported that the Iranian version of MDBQ has acceptable reliability and validity. In their study, the reliability of the factors ranged from 0.65 to 0.81 (17).
3.4. Tower of London Test
The Tower of London Test (TOL) was first introduced by Shalis (1982) to evaluate one of the brain's executive functions, and programming (which is sensitive to the function of the frontal lobe), and its computer mapping was designed in 1993 (18). In this study, computerized mapping of the test was used. Different stages of the test are displayed on the touch screen. On each screen, two layouts are shown to the experimenter, each of which has three vertical columns of different sizes and rings in three different colors.
The top layout is shown as a pattern or target to the experimenter and cannot be changed. The bottom layout consists of loops whose location can be changed by the experimenter. The rings move with the touch of a finger on a computer screen or using a mouse. This test has four stages, each of which gradually becomes more difficult than the previous stage. The first stage is solved with two movements, the second stage with three movements, the third stage with four movements, and the fourth stage with five movements. Each of the above four stages can be repeated four times.
After explaining the instructions, the experimenter is reminded to look at the top arrangement before starting each step and to consider the location of the rings. He can solve the problem with the least possible movement.
The final results that are recorded by the computer in each step are as follows: (1) the number of movements performed by the experimenter in each of the four sections of each step; (2) the programming time, which is the time interval between the presentation of the task on the screen and touching the first loop by the experimenter; (3) the time of the test, which is the time interval between touching the first loop and the end of the task.
Finally, the average number of movements, the average planning time, and the average thinking time are recorded and shown.
3.5. Wisconsin Card Sorting Test
The Wisconsin Card Sorting Test (WCST) is a well-known neurocognitive task used by clinical neuropsychologists to measure cognitive flexibility and aspects of executive functioning (19). There are manual and computerized forms.
The computerized version of WSCT was designed by Kimberg et al. (2000), in which the participants are asked to sort cards based on one of these variables: Color, number, or shape. Participants are unaware of sorting pattern, but by receiving feedback concerning their sorts, they should find the pattern. They continue sorting one card until eight correct sorts are done, and when the sorting criterion is changed, participants should sort cards in another way (based on another criterion) until the other eight correct sorts are made. The test ends after 288 sorts are done, or 15 sorting categories are made. When the sorting criterion is changed, the participant may fail to find out the new sorting pattern and insist on the previous one. Damage to the human dorsolateral prefrontal cortex (DLPFC) may cause an impairment in the WCST performance, leading typically to perseveration on the first classification. The dependent measure in WSCT is the number of these perseverative errors.
3.6. Reaction Time
This experiment, invented by Dunders (1868), was performed using a computer. Due to the limited facilities of the laboratory, only the visual reaction time was evaluated. The participants were seated in front of the LCD screen at the desired distance (2 me away from the monitor). The display panel has four colored buttons (red, green, yellow, and blue) on a gray background. Participants can press all four keys with their thumbs or the computer's mouse as soon as a color circle stimulus appears. Each stimulus was presented 5 s on the screen with intervals of 10 s between stimuli. Each was presented 30 times, and the respective averages were calculated. Participants were instructed to respond to the colored stimulus as quickly and accurately as possible. When the stimulus appeared, the timer was activated (with the accuracy of 1 ms) and deactivated when the subject responded by pressing the key (20).
3.7. Ethical Observations
The research protocol was confirmed by the research ethic committee of Shiraz University of Medical Sciences (code: IR.SUMS.MED.REC.1398.506), and all participants were free to leave the research. They also signed the informed consent and got enough insurance that the results were confidential.
3.8. Data Analysis
Data extracted from the questionnaire and tasks were analyzed by SPSS version 19 using descriptive statistics and Univariate analysis of variance. A P ≤ 0.05 was considered significant.
4. Results
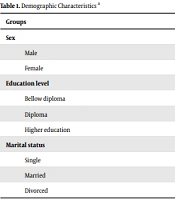
Demographic variables in the group with MDD and the control group are presented in Table 1 (the two groups were homogenous for these variables). The mean age of the subjects in the group with MDD and the control group was 38 ± 8 and 36 ± 9 years, with no significant differences (P = 0.23). The driving skill was measured in two groups; the number of car accidents during the last year was not statistically significant between groups (P = 0.55). Finally, we evaluated their driving skills using a seven-point Likert scale. All four subscales and the total score of MDBQ (Table 2) showed significant differences between the two groups. The mean MDBQ score in the MDD group was 2.5 times more than the control group. Based on the MDBQ scores, all participants were divided into risky and non-risky drivers; thus, 37% were depressed and risky, and 36% were non-depressed and non-risky drivers. The remainder of the analysis was then run, considering these four groups. A statistically significant difference was found between the groups in the reaction time test, WCST, and TOL test scores.
| Groups | Depressed | Non-depressed | P-Value |
|---|---|---|---|
| Sex | 0.92 | ||
| Male | 30 (59) | 11 (22) | |
| Female | 21 (41) | 39 (78) | |
| Education level | 0.088 | ||
| Bellow diploma | 13 (22) | 5 (10.2) | |
| Diploma | 15 (29) | 10 (20) | |
| Higher education | 23 (45.1) | 34 (69) | |
| Marital status | 0.094 | ||
| Single | 16 (30) | 9 (18) | |
| Married | 20 (39) | 25 (50) | |
| Divorced | 15 (29) | 16 (32) |
a Values are expressed as No. (%).
| Variables | Mean ± SD | P-Value |
|---|---|---|
| Slips | 0.001 | |
| Depressed | 17 ± 9 | |
| Control | 7 ± 7 | |
| Deliberate violation | 0.001 | |
| Depressed | 15.05 ± 10 | |
| Control | 5 ± 6 | |
| Unintentional violation | 0.001 | |
| Depressed | 3.04 ± 2 | |
| Control | 0 ± 1 | |
| Lapse errors | 0.001 | |
| Depressed | 5 ± 4 | |
| Control | 2 ± 2 | |
| Total score | 0.001 | |
| Depressed | 40 ± 23 | |
| Control | 16.02 ± 16 |
4.1. Reaction Time
Comparing the mean reaction time between the groups represented that the highest mean reaction time was related to the depressed and non-risky drivers, and the lowest was related to non-depressed and risky drivers (Table 3).
| Group | Depressed Risky | Non-depressed Non-risky | Non-depressed Risky | Depressed Non-risky |
|---|---|---|---|---|
| Reaction time (Mean ± SD) | 690 ± 204 | 731 ± 176 | 597 ± 185 | 875 ± 398 |
a F= 3, P = 0.018.
4.2. WCST
According to the WCST scores, the highest number of trials administered and total errors were found in the depressed and risky drivers. The non-depressed and non-risky drivers showed the lowest score in all domains of WSCT. These differences were statistically significant (Table 4).
| Variables | Mean ± SD | F | P-Value |
|---|---|---|---|
| Trials to complete first category | 5 | 0.002 | |
| Depressed, risky | 34.05 ± 20 | ||
| Non-depressed, non-risky | 16 ± 15 | ||
| Non-depressed, non-risky | 25 ± 21 | ||
| Depressed, non-risky | 28 ± 23 | ||
| Total Errors | 5 | 0.001 | |
| Depressed, risky | 35 ± 8 | ||
| Non-depressed, non-risky | 27 ± 8 | ||
| Non-depressed, risky | 31 ± 9 | ||
| Depressed, non-risky | 33 ± 9 | ||
| Perseveration error | 4.09 | 0.009 | |
| Depressed, risky | 15 ± 9 | ||
| Non-depressed, non-risky | 9 ± 5 | ||
| Non-depressed, risky | 10 ± 6 | ||
| Depressed, non-risky | 14 ± 9 |
4.3. TOL
We utilized this computerized task to compare the groups regarding two main domains, including latency time (the time between presenting the stimuli and subject reaction) and the total time of the test. The depressed and non-risky drivers showed the longest mean latency time, while it was the shortest in non-depressed and non-risky drivers.
The mean total time of doing the task revealed that the non-depressed and non-risky drivers spent the shortest time doing the task and the depressed and non-risky drivers spent the highest time. The difference between the groups was significant. Table 5 presents the result of the univariate ANOVA.
| Variables | Mean ± SD | F | P-Value |
|---|---|---|---|
| Latency time | 11 | 0.001 | |
| Depressed, risky | 176 ± 97 | ||
| Non-depressed, non-risky | 86 ± 67 | ||
| Non-depressed, risky | 165 ± 56 | ||
| Depressed, non-risky | 213 ± 96 | ||
| Total duration | 16 | 0.001 | |
| Depressed, risky | 471 ± 217 | ||
| Non-depressed, non-risky | 219 ± 172 | ||
| Non-depressed, risky | 488 ± 191 | ||
| Depressed, non-risky | 609 ± 260 |
5. Discussion
Our results showed that the total score and the score of all MDBQ subscales were higher in the group with depression, which means that depression is negatively correlated with driving performance. Similar to our findings, previous studies have found that depression (independent of the severity) is associated with driving impairment (5, 12).
According to the mean total score of MDBQ in the depressed group (1.5 times more than in another group), depression is related to risky driving behavior. Scott-Parker et al. also showed similar results and reported that depression specifically predicted risky driving (21).
Pan et al. found that the mean reaction time of depressed patients was generally higher than normal controls, which may be an indicator of depression. They showed that these characteristics are statistically significant using the t-test (22). Similarly, in our study, the highest mean of reaction time was related to the depressed and non-risky drivers.
Also, in another study, cognitive impairment, decreased concentration, side effects of medications, suicidal thoughts/intentions, and reduced reaction time mostly influenced the fitness to drive decisions (23).
According to the WCST scores, the depressed and risky drivers had the worst function in comparison with another group (with a significant mean difference). WCST is a reliable neuropsychologic test to assess executive function. Executive functions encompass higher cognitive functions supporting goal-directed behaviors (24). Driving is a complex activity, which requires intact cognitive functions, including attention, visuospatial processing, and decision making. Depression can cause impairment in frontal cortex function, which leads to impaired executive functions (25).
In contrast to our findings, another study revealed that despite different drug treatments received by many patients, there was no significant difference in the driving task between patients with depression and healthy individuals (26). This can be due to different study populations. They recruited only partly remitted depressive patients who were under steady-state pharmacologic treatment, but in our study, samples were selected from inpatient and outpatient depressed individuals. The Performance on the WCST in MDD patients was significantly poorer in depressed patients (27).
The Tower of London task is another valid test to assess executive functions (specifically planning and problem-solving abilities) (28). Considering the mean scores of the Tower of London test, the non-depressed and risky drivers had the least latency time. Another study by Luciana showed that a shorter initiation time could reflect impulsivity and increased processing speed (29), which can explain why the latency time was shortest in risky drivers. The obtained mean scores revealed that the depressed and non-risky drivers had the longest time spent during this test. Consistent with our results, Moniz et al. found that the depressed patients spent a longer time significantly completing the Tower of London Test (30).
5.1. Limitations
The present study has several limitations. The small sample size was one of the limitations, which was attributed to the limited referral of patients with major depression to hospitals and clinics during the COVID-19 pandemic. The majority of cases were selected from inpatient individuals who were suffering from a more severe type of MDD. The healthy control group was restricted to the visitors and families of patients who were at a higher risk of mood disorders than the normal population. The depressed group was not screened for the severity of their disorders, and the control group was only assessed by a semi-structural interview rather than using psychometric tools.
The study population was not homogenous in gender, while the non-depressed females had the highest number. The driving performance may be different between males and females, which is preferred to be considered in future studies. Also, many patients were treated with drugs, which can be considered confounding factors.
5.2. Conclusions
In this study, we examined the correlation between driving performance and depression. The findings showed that depression might cause a significant decline in different aspects of driving behavior. An intact executive function is needed for driving safely as an important social activity. Depression is associated with deficits in multiple cognitive domains, such as executive function; thus, it can cause impairment in driving performance.
It should be considered that these findings are needed to be replicated in further studies, in which comorbidities and other confounders are addressed. Such improvements in understanding of the field can lead to the development of road safety policy recommendations for depressed patients. It is hoped that, by identifying the risk factors of collisions in depressed drivers and controlling them, psychiatry can play a significant role in reducing accidents and resulting mortality and their social consequences.