1. Background
Magnetic resonance imaging (MRI) and magnet field technology advancements and the increase in noninvasive neuroimaging techniques have led to the incidental detection of central nervous system (CNS) abnormalities. Many of these abnormalities are unidentified bright objects and have pathologies other than multiple sclerosis (MS). Some of these incidental abnormality findings in MRI of an asymptomatic individual that are strongly suggestive of MS are referred to as radiologically isolated syndrome (RIS) (1, 2). About 33% of individuals with RIS develop MS within five years of their first brain MRI (3).
Radiologically isolated syndrome is a recently-known condition with obscure associations with MS (4), and RIS patients may or may not progress to MS (3). There have been several studies suggesting that certain risk factors can contribute to its transition to MS (1, 3, 5, 6), including age, male gender, pregnancy, infratentorial and cervical lesions, the presence of neurofilament light chains, oligoclonal bands, increased CSF IgG indexes, and atypical visual evoked potentials (VEP) (1, 3, 4).
Diagnosis of patients with RIS provides the opportunity for the early commencement of disease-modifying therapy (DMT). This intervention can procure more time to prevent the advancement of in situ demyelination that may lead to clinical inflammatory events, radiological progress, and physical disability (5).
A subclinical application of optical coherence tomography (OCT) measurea of the thickness of different retinal layers and monitors the progression of visual pathway atrophy and neurodegeneration related to the progress of the entire brain atrophy and neurodegeneration (1, 4, 6).
Several studies have attempted to measure the thickness of the retinal nerve fiber layer, the ganglion cell layer, and the inner plexiform layer in the eyes of patients with MS with or without optic neuritis (ON) (3, 5).
2. Objectives
The purpose of this study was to measure the thickness of the ganglion cell layer (GCL) and macular retinal nerve fiber layer (mRNFL) layers of the retina by OCT in subjects with RIS and compare them with a healthy control (HC) group to assess the progression of visual pathway atrophy and neurodegeneration.
3. Methods
3.1. Study Subjects
This cross-sectional study was conducted in the MS unit of the Neurology Department of Kashani Hospital of Isfahan University of Medical Sciences. Medical records of patients who entered the study during 2019 and 2020 were investigated. Twenty-two patients with RIS and 23 healthy individuals as the control group were included in the study. Radiologically isolated syndrome was detected through the Okuda 2009 diagnostic criteria (7). Clinical and laboratory evaluations and MRI imaging were performed by an expert neurologist at the study location to confirm the clinical diagnosis and exclude other pathologies.
Visual pathologies such as glaucoma, retinal disease, corneal disease, severe ametropia or refractive error of ±6D or more, diabetes, age 60 or more, and ten patients were excluded from the study. The control group was matched with the RIS group in terms of age and gender.
3.2. Magnetic Resonance Imaging
Brain MRI examinations were conducted at different MRI units and included fluid- attenuated inversion recovery (FLAIR), T1-weighted, and T2-weighted sequences in coronal, axial, and sagittal view planes. Images were obtained by the Avanto Siemens 1.5 T scanner at various MRI centers. Individuals were included if the incidental anomalies seen in the white matter of the brain MRI met the 2009 Okuda criteria: (1) T2 hyperintensities measured > 3 mm and fulfilled Barkhof criteria for spatial dispersion (8); (2) corpus callosum, homogeneous foci, well-circumscribed, and ovoid were involved or not involved, (3) anomalous white matter in the CNS was not consistent with the vascular pathology (7).
3.3. Optical Coherence Tomography
Individuals were referred to Faiz Hospital to perform OCT. Swept-source OCT was used to obtain the OCT images (Topcon Japan and DRI OCT Triton) on both eyes of 47 participants by an experienced operator in Feiz eye hospital. The OCT examination was performed to evaluate mRNFL, ganglion cell layer, inner plexiform layer (GCIPL/GCL+), and GCL++ (mRNFL and GCL+) thickness. The mRNFL was calculated for the total thickness of inferior, superior, nasal, and temporal sectors. Also, the GCL+ and GCL++ were calculated for the temporal, nasal, inferior, and superior sectors.
An expert ophthalmologist confirmed all OCT scans after the procedure to ensure the quality exclusion of any potential retinal pathology.
3.4. Statistical Analysis
The SPSS (SPSS Inc, Chicago, IL, USA) was utilized for data analysis. The statistical and qualitative data were presented as mean ± SD, median (maximum, minimum), and frequency (percentage). Kolmogorov-Smirnov tests were performed to determine the normality of continuous data. Chi-square analysis was used for the comparison of qualitative data between groups. Based on the data, a t-test was conducted to compare the results between the groups. An analysis of statistical significance was performed using a P-value of 0.05.
4. Results
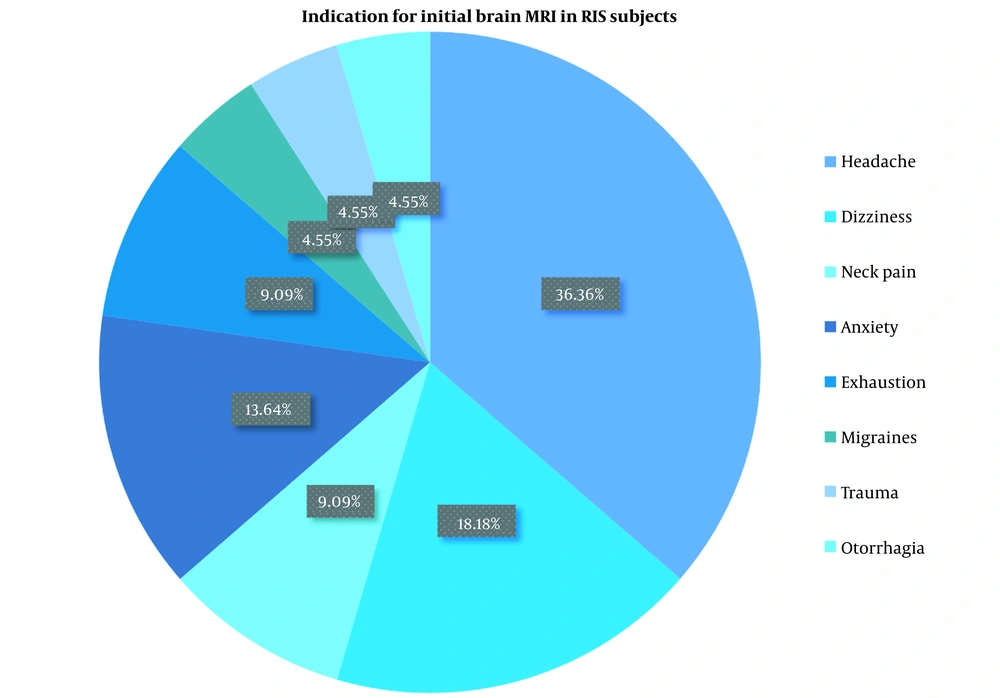
In total, 22 patients with RIS fulfilling 2009 Okuda criteria (7) and 23 healthy individuals were studied. Of these, 48.6% and 51.4% were women in RIS and HC groups, respectively. The mean ± SD age of RIS patients was 32.45 ± 6.07, and that of the HC group was 36.43 ± 9.95. Clinical characteristics and demographic profiles of the RIS and HC groups are presented in Table 1. The indications for the initial brain MRI in the RIS group varied among participants. The most commons were headaches and dizziness (Table 2). The indication for the brain MRI in the control group was headache in 17 and head trauma in 6 participants. The brain MRI findings were categorized based on their locations: Subcortical, cortical, or both. The findings were exclusively in the subcortical areas in 3 (13.6%) participants and in both subcortical and cortical areas in 19 (86.4%) participants (Table 3).
| RIS (n = 22) | HC (n = 23) | P-Value | |
|---|---|---|---|
| Subjects (eyes) | 44 (48.9) | 46 (51.11) | |
| Sex | 0.945 b | ||
| Female | 18 (48.6) | 19 (51.4) | |
| Male | 4 (50.0) | 4 (50.0) | |
| Age | 32.45 ± 6.07 | 36.43 ± 9.95 | 0.115 c |
| Median (min, max) | 33 (22, 47) | 39 (13, 54) | |
| CDVA (acuity), log MAR | 0.023 ± 0.06 | 0.008 ± 0.02 | 0.243 c |
| Median (min, max) | 0.90 (0.2, 1.0) | 0.90 (0.2, 1.0) | |
| Fundoscopy | - | ||
| Normal | 22 (48.9) | 23 (51.1) | |
| Abnormal | 0 (100.0) | 0 (100.0) | |
| Contrast acuity | |||
| Low contrast | 0.396 b | ||
| Lower than %50 | 17 (45.9) | 20 (54.1) | |
| Higher than %50 | 5 (62.5) | 3 (37.5) | |
| High contrast | - | ||
| Lower than %50 | 0 (0.0) | 0 (0.0) | |
| Higher than %50 | 22 (48.9) | 23 (51.1) |
Abbreviations: RIS, radiologically isolated syndrome; HC, healthy controls; SD, standard deviation; CDVA, corrected distance visual acuity.
a Values are expressed as No. (%) or mean ± SD unless otherwise indicated.
b Resulted from chi-square test.
c Resulted from independent t-test.
| Indication for Initial Brain MRI | No. (%) |
|---|---|
| Headache | 8 (36.4) |
| Migraines | 1 (4.5) |
| Dizziness | 4 (18.2) |
| Neck pain | 2 (9.1) |
| Anxiety | 3 (13.6) |
| Trauma | 1 (4.5) |
| Exhaustion | 2 (9.1) |
| Otorrhagia | 1 (4.5) |
Abbreviation: MRI, magnetic resonance imaging.
| Location of Brain MRI Findings | No. (%) |
|---|---|
| Subcortical | 3 (13.6) |
| Cortical | 0 (0.0) |
| Both | 19 (86.4) |
Abbreviation: MRI, magnetic resonance imaging.
4.1. Optical Coherence Tomography
The thickness of the mRNFL, GCIPL/GCL+, and GCL++ (mRNFL and GCL+) of 22 patients (44 eyes) with RIS was compared to that of 23 healthy individuals (46 eyes) (Table 4).
| Layer | RIS (n = 44) | HC (n = 46) | P-Value |
|---|---|---|---|
| mRNFL | |||
| Total thickness | 110.34 ± 13.71 | 112.10 ± 11.23 | 0.504 |
| Superior | 130.84 ± 25.92 | 138.26 ± 19.49 | 0.127 |
| Inferior | 146.20 ± 22.68 | 144.86 ± 17.18 | 0.753 |
| Nasal | 85.04 ± 14.94 | 86.13 ± 15.38 | 0.735 |
| Temporal | 75.72 ± 11.51 | 77.56 ± 10.64 | 0.433 |
| GCL++ | |||
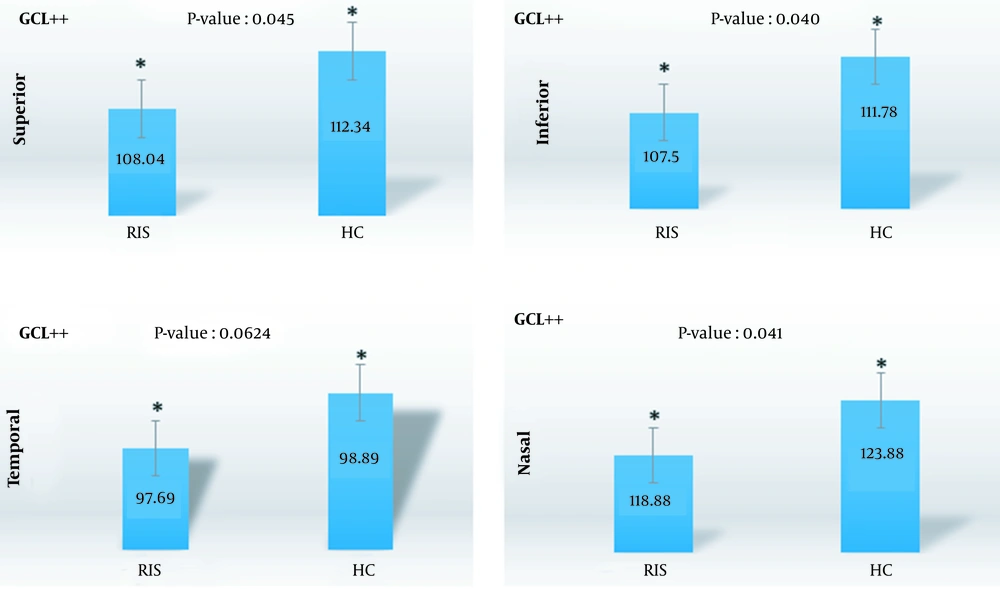
| Superior | 108.04 ± 12.43 | 112.34 ± 8.31 | 0.045 |
| Inferior | 107.50 ± 12.13 | 111.78 ± 8.00 | 0.040 |
| Nasal | 118.88 ± 14.76 | 123.88 ± 9.23 | 0.041 |
| Temporal | 97.69 ± 9.20 | 98.89 ± 13.39 | 0.624 |
| GCL+ | |||
| Superior | 73.22 ± 8.75 | 72.84 ± 6.56 | 0.816 |
| Inferior | 71.61 ± 9.19 | 72.00 ± 5.17 | 0.805 |
| Nasal | 75.85 ± 9.31 | 75.77 ± 11.32 | 0.971 |
| Temporal | 74.45 ± 8.29 | 74.76 ± 6.02 | 0.841 |
Abbreviations: RIS, radiologically isolated syndrome; HC, healthy controls; mRNFL, macular retinal nerve fiber layer; GCL+, ganglion cell layer and inner plexiform layer (GCIPL); GCL++, mRNFL and GCL+.
a Results from independent t-test.
The mRNFL thickness was lower in the RIS group than in the controls. However, no statistically significant difference was found (P-value > 0.05). Total mRNFL thickness was 110.34 ± 13.71 μm in the RIS group and 112.10 ± 11.23 μm in the HC group. Regional analysis of the mRNFL showed that the difference in thickness was more prominent in the superior quadrant, and the superior quadrant had the most prominent reduction in thickness (P = 0.127). In regards to GCL++ thickness, there was a statistically significant difference between the RIS and the HCs in the nasal (P = 0.041), superior (P = 0.045), and inferior (P = 0.040) quadrants. As shown in Table 4, the thickness of these quadrants was less in the case group. The nasal (P = 0.041) quadrant showed the highest reduction in thickness compared to other regions of the GCL++. Meanwhile, no significant reduction was seen in GCL+ thickness (P-value > 0.05). The difference between the two groups was not statistically significant when the retinal layer thickness of the right and left eyes of the RIS group were compared (P-value > 0.05) (Table 5) (Figures 1 and 2).
| Layer | OD (n = 45) | OS (n = 45) | P-Value |
|---|---|---|---|
| mRNFL | |||
| Total thickness | 112.00 ± 12.65 | 110.48 ± 12.37 | 0.568 |
| Superior | 135.33 ± 18.57 | 133.93 ± 26.97 | 0.755 |
| Inferior | 146.00 ± 19.42 | 145.04 ± 20.69 | 0.822 |
| Nasal | 88.04 ± 15.53 | 83.15 ± 14.40 | 0.125 |
| Temporal | 77.82 ± 11.09 | 75.51 ± 11.01 | 0.324 |
| GCL++ | |||
| Superior | 111.62 ± 10.67 | 108.86 ± 10.65 | 0.224 |
| Inferior | 110.26 ± 10.76 | 109.11 ± 10.11 | 0.601 |
| Nasal | 121.33 ± 12.65 | 121.54 ± 12.36 | 0.936 |
| Temporal | 100.35 ± 8.37 | 96.25 ± 13.72 | 0.091 |
| GCL+ | |||
| Superior | 73.88 ± 6.92 | 72.17 ± 8.34 | 0.293 |
| Inferior | 72.13 ± 7.42 | 71.48 ± 7.39 | 0.681 |
| Nasal | 76.61 ± 6.76 | 75.01 ± 12.99 | 0.466 |
| Temporal | 75.26 ± 7.26 | 73.95 ± 7.11 | 0.390 |
Abbreviations: mRNFL, macular retinal nerve fiber layer; GCL+, ganglion cell layer and inner plexiform layer (GCIPL); GCL++, mRNFL and GCL+.
a Results from independent t-test.
5. Discussion
It is demonstrated that RIS occurs in 0.1% of the general population (9, 10), more in patients with headaches, and in up to 2.9% of the relatives of patients with MS (9). It is estimated that 33% of patients with RIS develop MS within 5 years (10). From a retrospective perspective, RIS may be considered a preclinical form of MS.
Several longitudinal clinical studies on MS demonstrated a reduction in the mean, superior, inferior, and temporal retinal nerve fiber layer (RNFL) (11), the TRV, mRNFL, GCIPL (12) and the mGCIPL and peripapillary retinal nerve fiber layer (pRNFL) (13) thicknesses by OCT over time.
Previous studies have shown that RNFL thickness decreases over time in MS patients, with a gradually increasing trend. Previous studies have demonstrated that RNFL thickness is correlated with visual impairment, axon loss, brain atrophy, cognitive and physical disorders, and MRI abnormalities (14).
Moreover, studies on the eyes of patients with MS, with or without NO, have shown thinning of the GCIP and the RNFL (4, 8). There is evidence that GCIPL thinning in patients with MS becomes more noticeable with the progress of the disease, regardless of the individuals' ON history (13). Consequently, patients with MS have a reduced quality of life (15).
A study of children with clinically isolated syndromes (CIS) found that the number of ganglion cells, the inner plexiform layer, and the topography in CIS-non-optic neuritis (NON) eyes were significantly reduced compared to those with CIS-ON eyes (16). Optical coherence tomography is a non-invasive method of measuring the thickness of the retinal layers.
The present study aimed to evaluate the thickness of the mRNFL, GCL+ (GCIPL), and GCL++ (mRNFL + GCIPL) in people with RIS in Isfahan. According to our results, there were no significant demographic metrics differences in the study groups.
Based on our findings, despite a lower mRNFL thickness in the RIS patients compared with the controls, no statistically significant differences were observed (P-value > 0.05). In the RIS group, the total mRNFL thickness was 110.34 ± 13.71 μm, while in the HC group, this thickness was 112.10 ± 11.23 μm. Regional analysis of the mRNFL showed that the difference in thickness was more prominent in the superior quadrant, and the superior quadrant showed the most significant thickness reduction (P = 0.127).
The GCL++ (mRNFL + GCIPL) thickness demonstrated a significant difference between the RIS and the HCs in the nasal (P = 0.041), inferior (P = 0.040), and superior (P = 0.045) quadrants. Meanwhile, no significant reduction was seen in GCL+ thickness (P-value > 0.05).
The findings of our study are consistent with those of two cross-sectional studies investigating early MS patients and CIS (17). The authors of the first study found that, in patients in the early stages of MS, compared to HCs, there were reductions in GCIPL and mRNFL in the eyes with no ON of newly-diagnosed MS patients) (17). In the second study, the eyes of the patients with CIS without OP showed a lower GCIPL and a lower mRNFL compared with HC eyes (18).
The current study demonstrated that mRNFL thickness was lower in the RIS group than in the HC group. However, this difference was not significant statistically, which may be attributed to the limited number of subjects. However, the GCL++, which includes the three innermost retinal layers (mRNFL + GCL+ IPL), showed a statistically significant difference compared with controls. Many studies on patients with MS have reported a reduction in the GCL++ thickness and mentioned it as a ganglion cell complex (GCC) (19). The RIS group had thinner mRNFLs than the healthy group, according to this study. The limited number of subjects may have contributed to the lack of statistical significance of the difference.
A similar study by Vural et al., which examined retinal degeneration in RIS and its relationship with brain volume reduction and prognosis, confirms our findings. The RIS group comprised 15 patients, while the control group included 15 subjects. RIS significantly reduced GCIPL and mRNFL thickness in comparison with the control group. Mean differences of 28 μm and 30 μm in mRNFL thickness between the RIS and control groups, respectively, was noted, while the mean difference in GCIPL was 78.5 μm in the RIS and 80 μm in the HC groups (3).
In a similar study, Aly et al. investigated whether thinning of the inner retinal layer could predict conversion to multiple sclerosis in RIS. The group with RIS consisted of 36 participants, and the HC group consisted of 36 participants. The study subjects were followed for six years. According to the results, the RNFL thickness in the RIS individuals had a significant reduction compared to the HC. The mean thickness of RNFL was 98.8 ± 12.0 μm in RIS and 104.9 ± 7.7 μm in the control group, and the mean thickness of GCIPL was 1.99 (1.83 - 2.09) mm3 in the RIS and 2.04 (1.99 - 2.17) mm3 in the HC group (20). Eight patients with RIS progressed to MS. At the baseline and at follow-ups, there was a thinning of the pRNFL and the common GCIP of the patients who developed MS. In individuals with RIS, OCT can be useful to stratify the risk and make treatment decisions; and the reduction of the RNFL may be an independent risk factor for MS. This study supports the present study's results.
Our study has some limitations that should be discussed. The first limitation of this study was its cross-sectional design; therefore, no prognostic value for progression to MS can be confirmed. For this purpose, longitudinal and larger cohort studies of RIS with long follow-up periods are needed. Another limitation was that our study had a relatively small sample size, and possibly, in a larger study population, a more significant mRNFL and GCL++ thinning might be observed. Therefore, it would be plausible to expect that mRNFL and GCL++ atrophy may identify those at increased risk for progression to MS, but this remains to be confirmed.
5.1. Conclusions
Our study demonstrated that the mean thickness of mRNFL and GCL++ was less in the RIS subjects in comparison with the HC subjects, indicating that retinal neuroaxonal loss may be detected in RIS subjects. Based on these results, patients with RIS may benefit from OCT imaging for clinical monitoring. To confirm the role of retinal layer thickness in predicting clinical demyelination, design and implementation of prospective studies with larger sample sizes are warranted.