1. Background
Alzheimer disease (AD) is the most common cause of dementia (approximately 60% to 70% of all dementia cases) and cognitive impairment in the elderly (1, 2). In the United States, it was estimated that 6.7 million people aged 65 and older suffered from AD in 2020, which will increase to 13.8 million by 2060 (3). This number is expected to be 80 million by 2040 around the world (4). According to a recent neurophysiological and pathological study, synaptic plasticity loss is a significant feature of AD. Synaptic plasticity is essential for all complex cognitive functions, such as learning, abstract thinking, and memory (2). On the other hand, mild cognitive impairment (MCI) is considered a dividing line between cognitive changes in aging and the early stages of dementia (5). In general, MCI refers to a complaint of memory function in older people that is not detectable in clinical examination (6). Almost 50% of people with MCI develop AD within 5 years (7). Concern about cognitive changes (deficiency in at least 1 cognitive domain) and preserving independence in functional ability are diagnostic criteria for MCI. The clinical manifestations of this disorder differ based on the presentation of memory impairment or not (1).
Despite the great efforts to cure AD, there has been no significant success in influencing the course of the disease. One reason is that pathological changes begin decades before the appearance of clinical symptoms (2). On the other hand, identifying cognitive disorders in their early stages is an important challenge for physicians. Therefore, there has been a desire to make more accurate decisions about the early stages of the disease in recent years (5). Memory impairment is an early sign of AD, and atrophy of memory-related mesial temporal lobe (MTL) structures, particularly the hippocampus, is one of the first macroscopic signs of AD reported in neuroimaging studies (8). The MTL structures (the hippocampus, entorhinal, perirhinal, and parahippocampus) are critical in memory processing, particularly in encoding recently acquired information. On the other hand, in AD, these are the first areas to display a pathological change (9). In this way, hippocampus atrophy, as measured by magnetic resonance imaging (MRI)-based volume measures, can predict the progression to AD in older patients with MCI (4). On the other hand, neuroimaging studies have shown that the hippocampus is involved in preserving spatial information, as well as various levels of spatial working memory (SWM) processing (10).
Spatial working memory is an essential characteristic of the goal-oriented performance. Approaching a new situation demands a representation of the relevant spatial characteristics, needing to be constantly updated, maintained, and used when adaptive behaviors are required (11). On the other hand, working memory processes are involved in almost all high-level cognitive functions. As a result, hippocampal dysfunction leading to SWM deficits can interfere with a wide range of other cognitive skills (10).
In the first stage of AD, neurofibrillary tangles (NFT) spreads to one of the perirhinal cortical subsets. Several studies on monkeys have shown that the perirhinal cortex injury causes severe functional impairment of visual recognition memory tasks (12). Recollection and familiarity are 2 components of recognition memory (13). This type of memory is an important part of declarative episodic memory since it is distinguished by the inability to recall some things (14). Studies have shown that recollection is related to the hippocampus, whereas familiarity is related to the perirhinal cortex (13).
According to functional imaging findings, the hippocampus and parahippocampal gyrus play a role in associative aspects of memory. Studies have shown that the neural network required for paired-associate learning (PAL) includes the hippocampus and parahippocampal gyrus (15). Visual PAL is a type of episodic memory in which pairs of visual stimuli (such as shape and location) are encoded in memory; after that time, 1 stimulus is promoted to recall when exposed to other stimulus (16).
Egerhazi et al. and Cacciamani et al. examined these cognitive functions in people with MCI, people with AD, and normal individuals using the Cambridge Neuropsychological Test Automated Battery (CANTAB) and obtained different results (6, 17). Cacciamani et al. did not find significant differences between their study groups in any of the tests, but Egerhazi et al. showed a significant impairment in PAL and SWM in AD and MCI patients, while pattern recognition memory (PRM) was exclusively impaired only in the AD group (6, 17). It should be noted that Egerhazi et al. did not have a normal control group to be compared with (6). Considering the results of these studies, we do not yet have sufficient and convincing evidence about differences in cognitive functions, including SWM, recognition memory, and new learning between patients with AD, patients with MCI, and healthy people.
2. Objectives
We aimed to compare working memory, visual memory, and new learning in patients with AD, patients with MCI, and healthy people to find if there is any cognitive marker predicting MCI to AD.
3. Methods
3.1. Participants
A total of 15 patients with AD based on the National Institute on Aging and Alzheimer’s Association 2011 (NIA-AA 2011) criterion with a clinical dementia rating (CDR) score of 1 (mild stage), 18 patients with MCI based on the NIA-AA 2011 criterion with a CDR score of 0.5 (questionable stage), and 15 healthy people with a CDR score of 0 (no dementia stage) were included in this study. The patients were recruited from memory clinics of Tehran University of Medical Sciences. The participants’ ages were between 50 to 90 years old. They had no history of traumatic brain injury, major psychiatric disorders, vascular dementia, brain mass, or other neurodegenerative disorders (such as frontotemporal dementia, Parkinson disease, or Lewy body dementia) and orthopedic disorders (which prevent testing) based on their medical records. The subjects were excluded from the study if they refused to continue the study or were unable to take the tests. The 3 groups were matched on mean age, years of education, and gender distribution. The diagnosis was made based on clinical interviews and neuropsychiatric examinations by a neurologist (18).
3.2. Measures
3.2.1. Hamilton Depression Rating Scale
Hamilton Depression Rating Scale (HDRS) is a 17-item test used in semi-structured interviews to assess the severity of depressive symptoms (type and severity). This scale is one of the most widely used scales in clinical trials to assess depressive symptoms, and its validity and reliability have been confirmed. Of the 17 items, 9 items are scored from 0 (none) to 4 (severe), and the other 8 items are scored from 0 (none) to 2 (severe). The overall score is between 0 and 52; higher scores indicate more severe depressive symptoms. In this regard, 0 - 7 indicates no depression, 8 - 16 shows mild depression, 17 - 23 indicates moderate depression, and 24 and above indicates severe depression (19). The validity of the Persian translation of this tool was reported to be 0.55 and 0.39, respectively, through correlation with the Beck Depression Inventory and Ineffective Attitudes Scale. Its reliability was reported to be 0.95 among the evaluators (20).
3.2.2. Mini-mental State Examination
Folstein et al. as cited in Foroughan et al. developed the Mini-mental State Examination (MMSE) in 1975. In a psychometric evaluation of this instrument, Foroughan et al. showed good validity (α = 0.78) and reliability (α = 0.871; for the case group) in the elderly population using the Persian translation of this test (21). This test contains 30 questions that assess attention, orientation, memory, encoding, recalling, calculation, language, and the ability to draw a complex polygon. The presence of cognitive impairment is determined by the overall score. A cutoff score of 24/23 has been used to select patients with suspected cognitive impairment or dementia (22).
3.2.3. CDR
CDR was developed to demonstrate the phases of AD. It has also been used to detect MCI and distinguish it from normal aging and dementia (23). CDR is scored on a 5-point scale for each of the 6 cognitive categories of memory, orientation, judgment, problem-solving, social relationship, home and hobbies, and personal care. It is based on a semi-structured interview with the patient and a suitable companion. Zero indicates no disorder, 0.5 shows questionable impairment, 1 indicates mild impairment, 2 refers to moderate impairment, and 3 indicates severe illness. The overall CDR score is calculated using the scores of each person’s 6 categories as follows: CDR 0 = no dementia and CDRs 0.5, 1, 2, 3 = questionable, mild, moderate, and severe dementia, respectively (24). Mild cognitive impairment is measured on the CDR scale by a score of 0.5 (25). Cabral et al. and Perroco et al. used a CDR score of 0.5 as a valid score for MCI in their investigations (26, 27). The validity and reliability of this tool have been investigated in Farsi speaking people with a validity of 73% and reliability of 89% (Cronbach α coefficient) (28).
3.2.4. Cambridge Neuropsychological Test Automated Battery
Cambridge Neuropsychological Test Automated Battery was first developed in 1980 to measure cognitive functions in patients with dementia. This battery includes more than 20 tests that evaluate the following aspects of cognitive performance: Visual memory, executive functions, working memory, planning, attention, verbal and semantic memory, decision-making, response control, social cognition, and screening. Cambridge Neuropsychological Test Automated Battery has been used to distinguish between healthy adults and those with some disorders such as MCI, AD, attention deficit hyperactivity disorder, and a variety of central nervous system problems (29). The following subtests of CANTAB have been used in this study: (1) SWM that evaluates the subject’s ability to retain spatial information and manipulate them (6); (2) PAL that assesses visual memory and learning new information (30); and (3) PRM that evaluates visual recognition memory (31).
3.3. Procedure
The participants and their companions were first given a detailed explanation of the study process and then an informed consent form to sign. The participants were then evaluated using MMSE and HDRS. They were then asked to perform 3 subtests of CANTAB (SWM, PRM, and PAL). All participants were requested to complete their tasks by touching the screen while sitting in a comfortable chair placed about 0.5 m from the monitor. The tests were performed at the Neurocognitive Laboratory of Roozbeh Hospital, affiliated with Tehran University of Medical Sciences. The Ethics Committee of the Institute for Cognitive Science Studies approved the study (code: IR.UT.IRICSS.REC.1400.003).
3.4. Statistical Analysis
The differences between the 3 groups were measured in terms of gender using the chi-square and Fisher exact tests; other variables were analyzed by a 1-way analysis of variance (ANOVA) with a post hoc analysis to evaluate the differences between each 2 groups. Statistical analysis was performed using SPSS version 24 (SPSS Inc, Chicago, Ill, USA).
4. Results
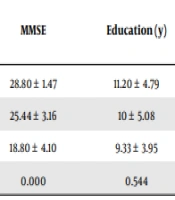
A total of 48 participants were recruited in 3 groups of AD, MCI, and healthy people. The differences between the groups in terms of age, gender, years of education, and depression score were not significant (Table 1). The participants differed significantly based on the MMSE score (P < 0.001).
| Groups | n | Age | MMSE | Education (y) | HDRS | Gender | |
|---|---|---|---|---|---|---|---|
| Male | Female | ||||||
| Normal | 15 | 64.26 ± 5.50 | 28.80 ± 1.47 | 11.20 ± 4.79 | 8.26 ± 4.72 | 5 | 10 |
| MCI | 18 | 68.22 ± 6.04 | 25.44 ± 3.16 | 10 ± 5.08 | 12.50 ± 6.78 | 8 | 10 |
| AD | 15 | 68.66 ± 4.15 | 18.80 ± 4.10 | 9.33 ± 3.95 | 9.26 ± 5.52 | 6 | 9 |
| P value | 0.053 | 0.000 | 0.544 | 0.100 | 0.931 | ||
Abbreviations: MMSE, Mini-mental State Examination; HDRS, Hamilton Depression Rating Scale; MCI, mild cognitive impairment; AD, Alzheimer disease.
a Values are expressed as mean ± SD.
The results of cognitive performance are as follows: Regarding SWM performance, there were significant differences between the 3 groups in terms of between errors and total errors (P = 0.000 and P = 0.001, respectively). Regarding PRM performance, there were significant differences between the 3 groups in terms of mean correct latency and correct number (P = 0.004 and P = 0.000, respectively). Regarding PAL performance, there were significant differences between the 3 groups in terms of first trial memory score, mean errors to success, and total errors adjusted (P = 0.000, P = 0.000, and P = 0.000, respectively). The results of the post hoc analysis (comparisons between pairs of groups) are presented in Table 2. The correlation analysis showed that the MMSE test scores were associated with all cognitive performance scores (P = 0.000), except for the strategy variable in SWM performance (P = 0.218). Table 2 shows the differences between the 3 groups in terms of cognitive performance.
| CANTAB Tests | Variables | AD | MCI | Normal | P Value | Comparison Between Each 2 Groups | P Value | Correlation with MMSE | |
|---|---|---|---|---|---|---|---|---|---|
| Correlation Coefficient | P Value | ||||||||
| SWM | Between errors | 78.86 ± 24.59 | 60.11 ± 17.22 | 48.86 ± 14.98 | 0.000 | Normal and MCI | 0.101 | -0.563 | 0.000 |
| Normal and AD | 0.000 | ||||||||
| MCI and AD | 0.008 | ||||||||
| Total errors | 82.26 ± 28.11 | 65.66 ± 18.10 | 52.33 ± 15.26 | 0.001 | Normal and MCI | 0.076 | -0.543 | 0.000 | |
| Normal and AD | 0.000 | ||||||||
| MCI and AD | 0.029 | ||||||||
| Strategy | 38.20 ± 9.29 | 39.94 ± 3.07 | 38.20 ± 3.48 | 0.348 | 0.181 | 0.218 | |||
| PRM | Mean correct latency | 4580.9 ± 1731.11 | 3271.4 ± 835.56 | 2855.2 ± 862.24 | 0.004 | Normal and MCI | 0.354 | -0.596 | 0.000 |
| Normal and AD | 0.007 | ||||||||
| MCI and AD | 0.037 | ||||||||
| Correct number | 14.86 ± 3.60 | 18.72 ± 3.10 | 20.93 ± 2.57 | 0.000 | Normal and MCI | 0.049 | 0.749 | 0.000 | |
| Normal and AD | 0.000 | ||||||||
| MCI and AD | 0.001 | ||||||||
| PAL | First trial memory score | 4.20 ± 3.00 | 12.50 ± 4.17 | 15.66 ± 3.26 | 0.000 | Normal and MCI | 0.015 | 0.812 | 0.000 |
| Normal and AD | 0.000 | ||||||||
| MCI and AD | 0.000 | ||||||||
| Mean errors to success | 8.52 ± 2.29 | 5.69 ± 2.98 | 3.82 ± 2.32 | 0.000 | Normal and MCI | 0.044 | -0.733 | 0.000 | |
| Normal and AD | 0.000 | ||||||||
| MCI and AD | 0.003 | ||||||||
| Total error adjusted | 156.00 ± 46.42 | 55.55 ± 42.16 | 28.73 ± 16.01 | 0.000 | Normal and MCI | 0.052 | -0.834 | 0.000 | |
| Normal and AD | 0.000 | ||||||||
| MCI and AD | 0.000 | ||||||||
Abbreviations: CANTAB, Cambridge Neuropsychological Test Automated Battery; AD, Alzheimer disease; MCI, mild cognitive impairment; MMSE, Mini-mental State Examination; SWM, spatial working memory; PRM, pattern recognition memory; PAL, paired-associate learning.
a Values are expressed as mean ± SD.
5. Discussion
Early detection of cognitive impairments (which lead to AD) can help clinicians to use early interventions to postpone the development of AD. This study was conducted to compare 3 memory-based cognitive functions (SWM, recognition memory, and new learning) between AD patients, MCI patients, and healthy people to find early cognitive impairments that can lead to AD.
The results indicated that the performance of SWM was not different between normal and MCI groups, while individuals with AD performed worse than the other 2 groups. In terms of recognition memory performance, normal people responded with more correct answers compared with the MCI and AD groups, and the MCI group performed better than the AD group. Regarding new learning skills, normal people had a better ability to learn new information compared with the other 2 groups. This ability was also better in MCI patients compared with AD patients. These results show that PAL and PRM can differentiate normal people from individuals with MCI and AD, as well as MCI individuals from patients with AD. Based on these results, SWM cannot differentiate well between MCI and normal groups, although the performance of patients with AD was significantly worse than that of healthy people. It can be concluded that we can use PRM and new learning abilities to track transferring from normal cognitive performance to minimal cognitive impairment and AD. The findings of this study are comparable to those of previous studies.
In 2 studies, Kessels et al. investigated spatial working memory. In one of them, they used the Box task to compare young people, the elderly, and persons with MCI (32). The results revealed that the MCI and the older groups did not differ in the visuospatial sketchpad, but the MCI group performed worse in the between-search error. This means that they cannot hold information for a longer period (32). In the other study, they measured working memory in individuals with MCI and AD using the Wechsler batteries and Span task. Their results showed that, unlike people with AD, people with MCI did not have a deficit in spatial span. However, another visual spatial working memory test revealed deficits in both groups (33). The results of these studies on spatial working memory ability in individuals with AD are consistent with the findings of our study.
Several studies on recognition memory have been conducted in these groups. Algarabel et al. showed that recollection decreases due to aging and neurological disorders, but familiarity is not affected by age (34). However, it can be impaired in those with MCI (34). Westerberg et al found that forced-choice recognition was normal in patients with MCI. From the anatomical viewpoint, neuropathology in hippocampus and entorhinal cortex (known to be present in MCI), affect recollection but not familiarity-based recognition (35). However, Wolk et al. revealed that patients with MCI had deficits in both recollection and familiarity (36). They stated that “our measure of familiarity was strongly associated with atrophy in AD-signature regions of the cerebral cortex.” They also showed a correlation between the measure of familiarity and AD biomarkers in groups with MCI and normal people (36). The findings of Algarabel et al. and Wolk et al. are consistent with those of the current study, in which we examined the familiarity aspects using the number of correct answers (34, 36).
There is little difference between the results of prior studies in the field of new learning. Harel et al. found that the MCI group had more total errors than the control group in the continuous PAL task (37). Consistent with our findings, Nanda et al. showed that in the free recall performance during the face-name paired continuous learning test, there were significant differences between the MCI and control groups, as well as between the MCI and AD groups (38).
Cacciamani et al. also compared the performance of MCI individuals with healthy individuals using SWM, PRM, and new learning tests of CANTAB. There were no significant differences between the groups (17). Further, Egerhazi et al. found that while recognition memory was impaired in AD (compared to MCI), both AD and MCI individuals showed poor performance in PAL and SWM tests (6). However, they did not have a normal control group to be compared. Campos-Magdaleno et al. also assessed visual memory in MCI patients at baseline and twice in follow-up using CANTAB. In their study, which is similar to the present study in using both PAL and PRM subtests, the results showed that visual memory evaluation using CANTAB could be useful for differentiating between different stages of MCI during its progress toward dementia (39).
The findings of the current study are not consistent with the results of Cacciamani et al., but they do agree with the results of Egerhazi et al. in PAL and Campos-Magdaleno et al. in both PRM and PAL (6, 17, 39).
5.1. Limitations
This study needs to be considered in light of some limitations, including a small sample size that can influence the generalization of its findings. The evaluation of the participants was limited to 3 memory tests. It is suggested that a larger study with some other neurocognitive tests can differentiate better the groups of AD, MCI, and healthy people.
5.2. Conclusions
The assessment of new learning function and recognition memory can be used as indicators of MCI and the progression of this disorder toward AD, whereas the assessment of spatial working memory function can only be used to assess the progression of MCI to AD.