1. Background
Glioblastoma (GB) is the most common and most aggressive primary central nervous system (CNS) tumor in adults (1), with an incidence of 4.32 /100,000 in the United States (2, 3), from 2.0 in Finland to 3.3 in North America, Austria, and Israel, and 4.96 per 100,000 in Gironde (1, 4). This incidence varies by age, sex, race, and region (5).
According to recent studies, the epidemiology and quality of life of patients with glioblastomas in the North African region are still little explored (6, 7). The incidence of glioblastoma can vary according to the socioeconomic level of the countries, with a higher rate in the western world than in low-income countries (8); this could be due to the very limited access to health services, the underreporting of glioma cases, and differences in diagnostic techniques and practices (9, 10). The current standard of care includes surgical resection at maximum, followed by concurrent and adjuvant radio-chemotherapy (11). The median survival from glioblastoma is approximately 1 to 2 years (8, 12), and the 5-year survival rate is approximately 5 to 9% (1, 13, 14). Prognostic factors for overall survival (OS) are patient age, patient functional capacity, grade of surgical resection, MGMT gene promoter methylation status, and type of treatment used (15-20).
2. Objectives
In Morocco, the survival of patients with glioblastoma is relatively little explored. In this sense, the present research aims to study the overall survival and these prognostic factors in patients with glioblastoma in the southern region of Morocco.
3. Methods
This is a retrospective cross-sectional study, and the data were extracted from the files of patients with glioblastoma in the public reference oncology center in the southern region of Morocco; it is a prognostic study including all patients living with glioblastoma cancer between January 2014 and October 2021.
3.1. Data Collection
A data collection sheet was used to collect the following variables from patient records: Age, sex, date of diagnosis, type of surgery (biopsy, partial surgery, complete surgery), and type of treatment (radiotherapy, concomitant radio-chemotherapy).
The (a) eloquent zone refers to the parietal, temporal, hypothalamus, thalamus, parieto-occipital, temporo-parietal, fronto-temporal, fronto-parietal, basal ganglion; (b) the non-eloquent area refers to the frontal and occipital lobes.
The vital status of patients was researched at the level of patient care structures via their attending physician (from medical records or by telephone contact) and from data from the city's death register, which records all cases of the deceased. Overall survival was calculated from the date of surgery to the date of patient death.
3.2. Statistical Analysis
Kaplan Meier's method was used to estimate patient survival, and the Log Rank test was used to compare results between different classes. The Cox model was used to study the factors associated with survival. Variables significantly associated with survival in univariate analysis with a P-value < 0.25 were introduced into the multivariate model. Relative risks (RR) were calculated with a 95% confidence interval (CI). The significance level was set at 0.05. Statistical analysis of the data was performed using SPSS 13.
4. Results
4.1. Patients and Treatment Characteristics
The total number of patient records was 82; the present study ultimately included 71 cases diagnosed with glioblastoma. 11 patients were excluded from the study; two patients with spinal involvement, four patients with incomplete data, and five patients were lost to follow-up. The median age at diagnosis was 57 (range 17.0 - 80.0 years), with a sex ratio of 1.44. The most common tumor region was the eloquent area (83.1%). Regarding the types of surgery, 29 patients underwent partial tumor removal, 26 cases had tissue biopsy, and 16 patients with total tumor removal. For the other treatment modalities, 42 cases were treated only with radiotherapy; on the other hand, 29 other patients received concomitant radiotherapy. Most patients received standard Radiotherapy treatment of 60 Gy for the dose administered during radiotherapy, while 29.6% of cases received hypofractionated treatment; these patients are all over 60 years of age (Table 1). Regarding the time between the surgical act and the start of radiotherapy, almost all patients (91.5%) started the first radiotherapy sessions only after six weeks of the operative act (Table 1).
| Characteristics | Values |
|---|---|
| Age group | |
| < 60 years | 42 (59.2) |
| ≥ 60 years | 29 (40.8) |
| Median age (range); y | 57 (17 - 80) |
| Gender | |
| Male | 42 (59.2) |
| Female | 29 (40.8) |
| Geographic origin | |
| Urban | 32 (45.1) |
| Rural | 39 (54.9) |
| Tumor location | |
| Confined to a single lobe | 38 (53.5) |
| Involved more than one lobe | 33 (46.5) |
| Tumor location | |
| Eloquent area | 59 (83.1) |
| Non-eloquent area | 12 (16.9) |
| Type of surgery | |
| Biopsy | 26 (36.6) |
| Partial tumor removal | 29 (40.8) |
| Total tumor removal | 16 (22.5) |
| Modalities of treatment | |
| RT | 42 (59.2) |
| CRCT | 29 (40.8) |
| Total dose (radiotherapy) | |
| Hypofractionated RT | 21 (29.6) |
| Hyperfractionated RT | 50 (70.4) |
| Days from surgery to initiation of RT | |
| ≤ 42 days | 6 (8.5) |
| ≥ 42 days | 65(91.5) |
| Overall survival | |
| < 9 months | 28 (39.4) |
| ≥ 9 months | 43 (60.6) |
Abbreviations: RT, radiotherapy; CRCT, combined radiotherapy and chemotherapy.
a Values are expressed as No. (%) unless otherwise indicated.
4.2. Survival Outcomes
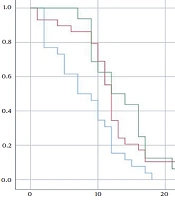
The median survival time (MST) of all GB patients in this study was 11 months (95% CI: 9.96 to 12.03 months) (Figure 1). Overall survival at two years is estimated at 4.2%.
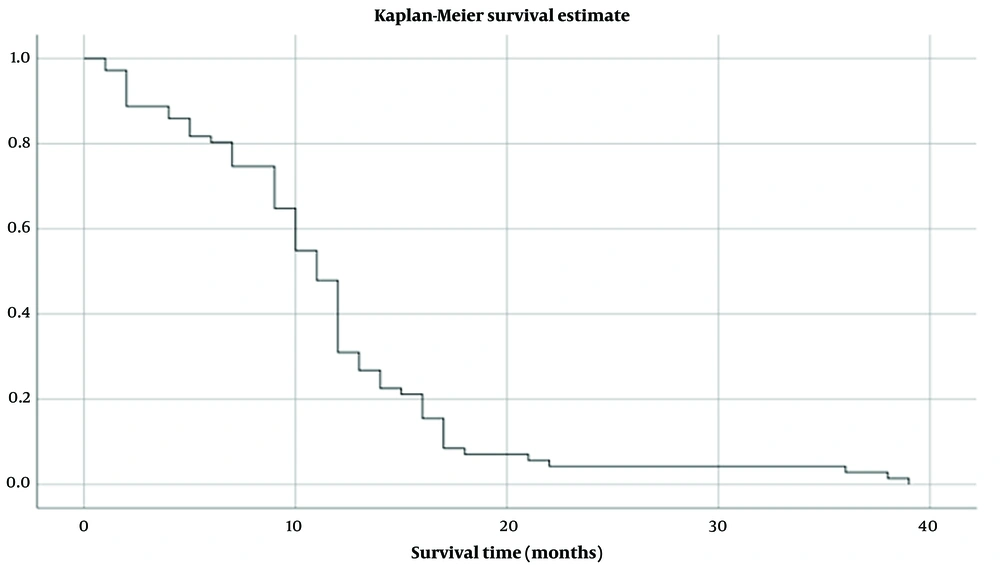
Using Kaplan-Meier, the results demonstrated a median overall survival of 12 months in patients with an age of fewer than 60 years, compared to only seven months in patients aged ≥ 60, with a significant difference between the two groups (P = 0.007) (Figure 2). Regarding gender, men showed a median overall survival of 12 months; on the other hand, in women was only ten months, with a non-significant difference between the two groups (P = 0.097).
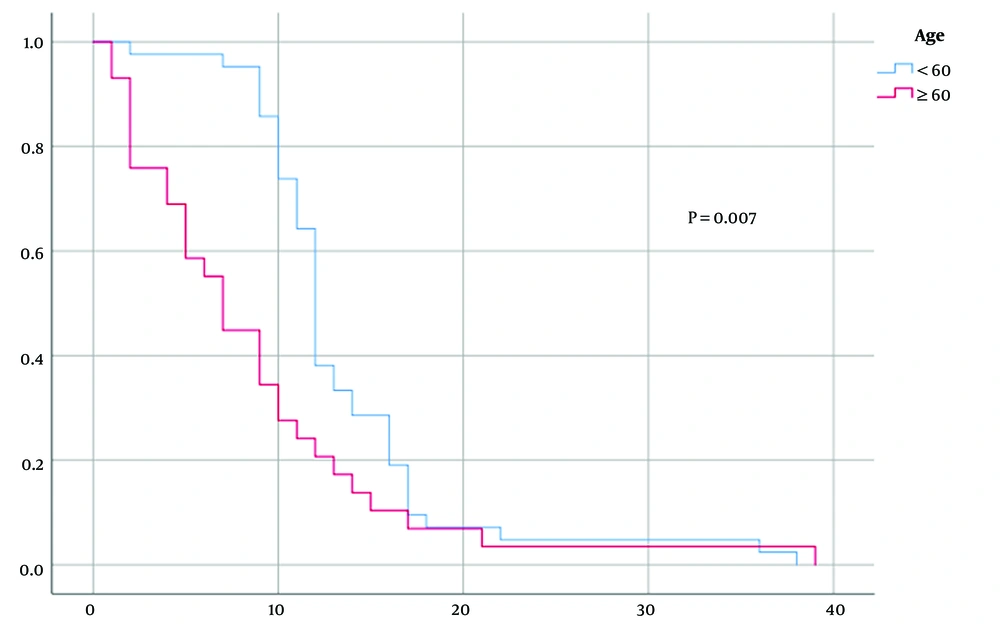
However, geographical origin demonstrated a significant difference between patients with a higher median overall survival in rural patients (median survival 12 vs. seven months, P = 0.001) (Figure 3). On the other hand, according to the eloquent localization of the tumors, the results of the present study did not reveal a statistically significant difference in survival between the groups (P = 0.373).
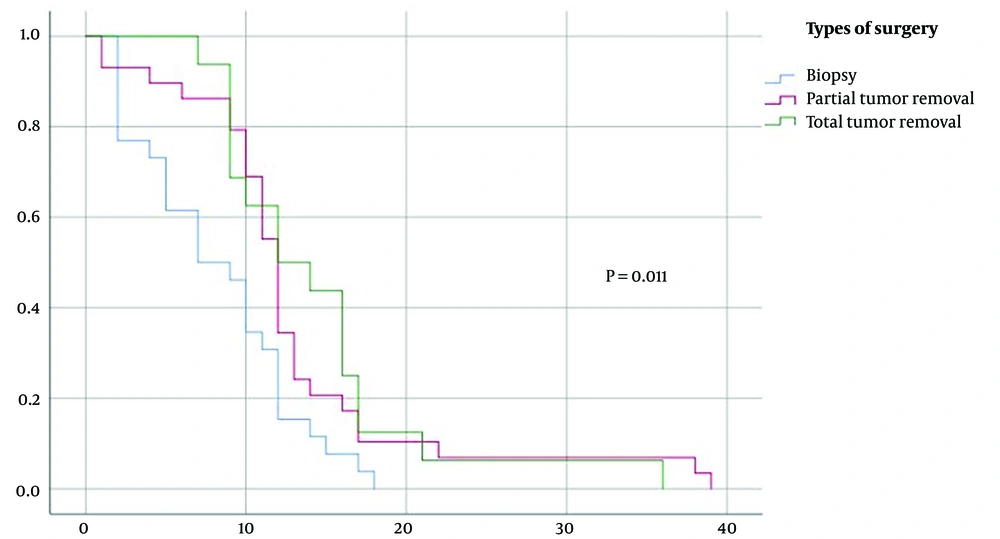
Compared to surgery, patients who underwent complete or partial tumor resection demonstrated a higher median survival of 12 months compared to 7 months for patients with biopsy, with a significant difference between the 3 three groups (P = 0.011) (Figure 4).
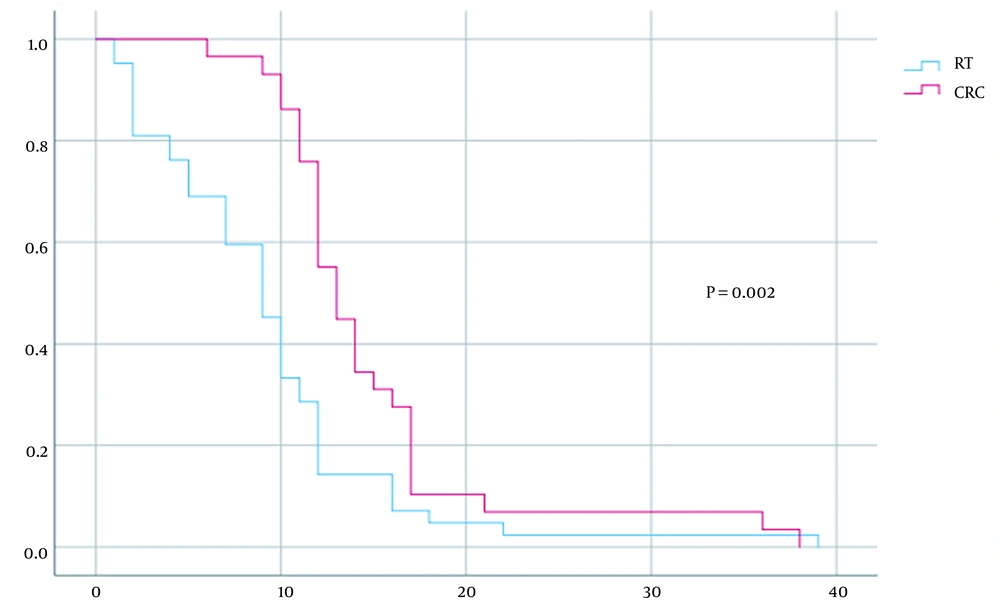
For the type of treatment used for each patient, the group treated only with radiotherapy demonstrated significantly reduced survival compared to patients treated with standard RCC treatment (median survival of nine versus 13 months, P = 0.002) (Figure 5).
4.3. Prognostic Factors
The COX regression model has been used to study the impact of prognostic factors on overall survival. Initially, the univariate analysis examined the sex, age, geographical origin of the patient, and type of surgery.
The results showed that gender, age, geographical origin, type of treatment, and type of surgery were significant at a level of P = 0.20 (Table 2). They are then included in the multivariate model.
| Variables | HR | P-Value |
|---|---|---|
| Age | 1.814 | 0.014 |
| Gender | 1.456 | 0.126 |
| Tumor location | 0.770 | 0.415 |
| Origin geographic | 0.327 | 0.001 |
| Type of surgery | 0.688 | 0.017 |
| Type of treatment | 0.499 | 0.005 |
Abbreviation: HR, hazard ratio.
Hazard ratios (HR) of multivariate results are shown in Table 3. After adjusting for all factors, the results revealed that only gender, age, and geographical origin were statistically significant predictors of overall survival. There is a tendency towards significance for the type of treatment.
| Variables | HR | 95% CI | P-Value |
|---|---|---|---|
| Age | 1.912 | 1.150 - 3.181 | 0.012 |
| Gender | 1.847 | 1.105 - 3.087 | 0.019 |
| Origin geographic | 0.371 | 0.212 - 0.650 | 0.001 |
| Type of surgery | 0.895 | 0.633 - 1.264 | 0.528 |
| Type of treatment | 0.619 | 0.366 - 1.048 | 0.074 |
Abbreviations: HR, hazard ratio; CI, confidence interval.
5. Discussion
Glioblastoma is the most aggressive primary tumor of the central nervous system. Despite recent therapeutic advances, patients with glioblastoma have a poor prognosis, with a median survival that does not exceed 15 months after diagnosis (15, 21).
The results of our study revealed a male predominance, which agrees with several previous studies (22-24). The median Overall Survival in our series was 11 months (95% CI: 9.96 to 12.03 months) which is consistent with other studies by Lacroix et al. (10.6 months) (17), Laws et al. (10.2 months) (18). The overall survival at two years was 4.2% which is lower than the rates found in some studies in developed countries (21.3% to 27.2%) (14, 25); this could be explained by the presence in these countries of more developed screening programs and diagnostic and treatment infrastructure.
For elderly patients with glioblastoma, results showed poor overall survival compared to patients younger than 60, consistent with several previous studies that have shown the effect of age as a factor influencing the survival of patients with glioblastoma (17, 18, 26, 27).
According to a recent meta-analysis, the effect of radiotherapy wait time on survival in patients with glioblastoma is still controversial (28); this is difficult to verify in the present study, as the majority of patients started radiotherapy only after six weeks of surgery, which can be explained by the long waiting time due to the lack of treatment facilities. The results of our study indicated that geographical origin is a predictor of overall survival, with better overall survival observed in rural patients compared to urban patients, which might be explained by the environmental factors of patients from rural areas; future studies will be necessary to confirm and deepen these findings.
Regarding the type of surgery, significant results show that the extent of resection can improve the overall survival of patients; this result is supported by univariate regression, which shows that the extent of resection is a factor in overall survival, which is in line with several studies showing that a better maximum resection is a predictor of increased survival for patients with glioma (17, 29-31). In addition, the type of post-surgical treatment has an impact on the survival of the patients in our series; the standard treatment with radio-chemotherapy concomitantly with temozolomide offers favorable survival results compared to patients treated only with radiotherapy, which in turn results in agreeing with the results of the literature (15, 32).
The present study had several limitations, in particular its retrospective design, with missing data in the patient files such as the Karnofsky Index (KPS) and molecular biology data, namely, the IDH mutation and the methylation of the MGMT promoter, which are considered to be prognostic factors in patients with glioblastoma.
5.1. Conclusions
The survival rate in patients with GB is improved with surgery, followed by concomitant radio-chemotherapy with temozolomide. We also confirmed that age and gender are important prognostic factors for the survival of GB patients. Furthermore, the data suggest the effect of the geographical origin of the patients on the overall survival of the patients as the only modifiable prognostic factor; indeed, future prospective studies are recommended to prove this result.