1. Introduction
Millions of people around the world are suffering from addiction to psychostimulants, alcohol, nicotine, and opiates (1), which impose great economic costs on individuals and society (2). Today, researchers describe addiction as a disorder with molecular and physiological changes and identify various environmental, genetic and neurobiological factors involved in it. In this vein there are so many different and long-term treatment methods and most of them have already been ineffective (3, 4). Prolonged drug exposure causes short-term but stable changes in the performance of opioid-sensitive neurons by stimulating adaptive mechanisms, such as sensitization, dependence and tolerance development (5). These changes cause high vulnerability of addicted people, even for years after withdrawal (6). Thus, these permanent brain changes and the interaction between opioid drugs and synaptic plasticity in different brain regions enhance the risk of relapse (3, 7). Drug dependence is an adaptive state when neurons adapt to repeated drug administration and may develop in both human and animals. Furthermore, this phenomenon is associated with behavioral manifestations rooted in the biological system (8) and classified as physical and psychological dependence (9). The reward system is responsible for psychological drug dependence, characterized by drug seeking behaviors and repeated drug abuse (10, 11). Psychological dependence is considered as the main cause of relapse (12).
CPP is a behavioral approach, which is extensively used to evaluate reinforcing and reward effects of different drugs such as opioids on rats (13). To be more specific, in CPP, animals are trained to associate a specific environment with drug and another environment with placebo and when they are free to choose between these two environments, they spend more time in the place associated with the drug (14). In the current study, CPP is used to evaluate morphine-dependence.
A substantial body of literature has suggested that exercise has a significant effect on preventing and treating a wide range of diseases. It has been found that exercise can alter various neurotransmitters’ activities and activate some of pathways, which are activated by morphine or other opiates; and also affects the reward system by releasing various mediators (15). Numerous experimental and clinical studies have demonstrated that chronic regular exercise can activate the central opioid system and cause increased release of endogenous opioid peptides and increase pain threshold in human and animals (16, 17). On a related note, exercise can influence the brain’s pleasure center through opioid systems and releasing neurotransmitters (18), and lead to secretion of specific neurotransmitters, which relieve mental and physical pain (19). Moreover, the brain can generate endogenous opioid-like compounds. The factors that increase these compounds in the brain will have the same effects as morphine and other opioid receptor agonists (20-22). Exercise increases the pain threshold by releasing androgen opioid peptides, especially Beta-endorphin (23-25). It is important to note that exercise can counteract the reduction in catecholamine’s production (dopamine, serotonin, and norepinephrine) following drug abuse. Therefore, exercise is considered as an effective treatment for drug abuse (23, 26).
In general, physical activity attenuates morphine dependence. Therefore, this natural reward system can replace other approaches to medication for addictive disorders (4) or use a prevention method to drug addiction (27). Exercise can be voluntary or compulsory, voluntary exercise causes no stress in animals and prevents negative effects of corticosterone on other neuronal circuit (28). Animal voluntary exercise is similar to that of human beings because in this type of exercise animals are able to regulate running speed, time, and distance (29). Moreover, stress has a significant effect on the relapse in both human and rodent models (7). More broadly, there are identified molecular mechanisms regarding the effect of voluntary exercise, which include its positive impact on enhancing neurogenesis, dendritic spines and presynaptic vesicles development, gene expression involved in synaptic plasticity in hippocampus and other brain region, increase in Long-term potentiation (LTP), and BDNF level (30). On the other hand, previous findings indicate that short-term compulsory exercise increases dependence symptoms following naloxone injection and decreases dependence symptoms in addicted animals (4).
Given that substance abuse is considered as one of the most challenging public health problems, it is critical to know how the substance abuse is and find new effective treatments. Also given the well-known beneficial effects of physical activity, voluntary exercise may be a beneficial method on protecting the central nervous system from the drug-related decline (4). Previous research demonstrates that physical activity, as a natural reward method, decreases morphine dependency, thus it can be replaced by other methods of treatment and also help addiction prevention. To better understand the role of compulsory and voluntary exercise on morphine CPP, the present experiment compared the effects of compulsory and voluntary exercise on CPP in male rats. Additionally, in studies examining CPP expression, both locomotion activity and weight change may influence the results, thus to determine if conditioned stimulus is the main determinant in locomotory response to morphine, locomotion activity and weight change were monitored in the current experiment. However, it was hypothesized that voluntary and compulsory exercise decrease CPP in male rats and there is no significant difference between weight changes and locomotion activity of rats designated in four experimental groups (compulsory, voluntary, morphine, and control groups). To the best of our knowledge this is among the first to compare simultaneously the effects of these 2 types of exercises on morphine-induced CPP in rats. Hence, in addition to examining the effects of voluntary and compulsory exercises on morphine-induces CPP in male rats, we compared the effects of these 2 types of exercises.
2. Methods
2.1. Animals, Experimental Treatments
Adult male Sprague-Dawley rats (n = 32; weighing 250 – 300 g), approximately 2 months of age, were proliferated and trained by the Center of Comparative and Experimental Medicine (Shiraz, Iran). Rats were kept under controlled conditions (temperature 21 ± 2°C, humidity 40% - 60% and light/dark cycle 12/12 hour (lights on at 7:00 am to 19:00 pm) and had access to food and water Ad-libitum. The research protocol was in accordance with the international principles for biomedical research involving animals. The procedure involving rats and their care was confirmed by the Shiraz University of Medical Sciences, animal care and use committee. Additionally, care was taken to use the minimum number of rats possible. We minimized the discomfort and pain of animal subjects and terminated the rats life with minimal pain and as quickly as possible.
Before the experiment, the rats were screened. Inactive rats were excluded and replaced by active ones. Then, selected rats were randomly divided into four groups as follows:
1) Compulsory exercise group: these rats were forced to run on a wheel with a constant speed of 11 meters per minute, 90 minutes per day for 14 successive days (4). On the 16th - 18th days of the experiment (after the exercise period), rodents received intra peritoneal saline injection as well as intra peritoneal morphine injection once a day (at 9:00 am and 3:00 pm) in the CPP stage.
2) Voluntary exercise group: these rats were housed in cages equipped with a running wheel with free access for 14 days. Wheel turns were measured with a micro-switch so that only complete 360 degrees turns were recorded. Wheel running was voluntary and the rodents had to run at least 100 meters per 24 hours to show the effects of exercise (31). On the 16th - 18th days of the experiment following the exercise period, rats received intra peritoneal morphine and saline injection once a day in the CPP stage.
3) Morphine group: this group did not perform exercise but did receive morphine and saline injection in the CPP stage. Theses rodents were housed in their cages without any intervention during the first 14 days of experiment and on the 16th-18th days of experiment (at 9:00 am and 3:00 pm) they received intra peritoneal morphine and saline injection once a day in the CPP stage.
4) Control group: this group neither exercised nor received morphine in the CPP stage. The rats were initially housed in their cages without any intervention for 14 days. Then, in the CPP stage they received normal saline solution through intra peritoneal injection twice a day (at 9:00 am and 3:00pm) on the 16th - 18th days of the experiment.
2.2. Conditioned Place Preference (CPP)
The CPP device was composed of 2 equal-sized rectangular chambers (44.5 × 16.5 × 34 cm) and a central chamber (44.5 × 11 × 34 cm), which were accessible through sliding valves (32). The floors of the chambers were separated by sensory stimuli; 1 of the chambers had a rough floor with walls shaded vertically, another chamber had a soft floor with walls shaded horizontally. The unbiased CPP procedure was used in this study, which lasted 5 days (33). This 5-day schedule of conditioning included the following stages: preconditioning, conditioning, and post conditioning.
2.3. Preconditioning Stage
On the 1st day of the experiment, after opening the sliding valves, each rat was placed in the central chamber for 10 minutes and was allowed to move freely throughout the box. The duration of the presence for each rat in different parts of box was recorded by chronometer (in seconds) to determine the less preferred place (33).
2.4. Conditioning Stage
This stage consisted of 3 successive days as follows (2nd - 4th): In the conditioning (CPP) stage, the voluntary exercise group, the compulsory exercise group, and the morphine group received morphine injection and saline injection once a day (9:00 am and 3:00 pm). The control group also received saline injection twice a day. CP was conducted for 3 days (twice a day). On the 2nd day of the CPP, each rat received 10 mg/kg morphine injection (intra peritoneal) in the morning and was immediately placed in less preferred chambers of the CPP device with closed sliding valves for 30 minutes. Rats also received 10 mg/Kg saline injection (intra peritoneal) in the afternoon and were immediately placed in the other chamber of the CPP device with closed sliding valves for 30 minutes. On the 3rd day, the morphine and saline was increased to 20 mg/kg and morphine and saline were injected in the reverse order of the 1st day. That is, they received saline in the morning and morphine in the afternoon. The injection dose on the 4th day was the same as the 2nd day and the morning and afternoon injection turns were the same as the 1st day. The injection dose and turn for the control group was like the other 3 groups except that normal saline solution was injected twice a day. Injections were performed in the morning and the afternoon in order to avoid round-the-clock changes during the CPP (34, 35).
2.5. Post Conditioning Stage
On the 5th day of CPP, each rat was allowed to move freely in the 3 chambers for 10 minutes (the sliding valves were opened). The duration of presence for each rat in each part of the CPP device was recorded by a video camera and was compared to the pre-conditioning stage (36-38).
Change in preference was calculated by subtracting the time spent in the less preferred part of the device (in which the rat received drug) on the test day from the time spent in this part in the preconditioning stage. This change in preference was expressed as mean ± standard deviation. Data analysis was carried out using the SPSS software version 20, one-way analysis of variance. In case there was a significant difference, data was analyzed using the Tukey test and P < 0.05 was considered as significant (33). To this end, the floor of lateral parts was divided into four equal squares by drawing a + sign in order to evaluate the rats’ locomotion activity (32). The locomotion activity of the experimental group was compared with that of the control group to determine if conditioned stimulants are the main determiners in loco motor response to morphine.
2.6. Voluntary Exercise
Rats in this group were housed in cages equipped with running wheels (33 cm thick, 9/5 cm wide) to which they had free access for 14 days. The number of wheel rotations was recorded by an automated system connected to the cage. In order to observe the exercise effects, the rodents must have run at least 100 m per 24 hours (32). The running distance was calculated according to the following formula: D = N × 2ηr in which D is the running distance, N is the number of wheel turns in 24 hours, η is 3/14, and r is the wheel radius.
2.7. Compulsory Exercise
Rats in this group were housed in cages with a compulsory wheel (33 cm thick, 9/5 cm wide). They were exposed to compulsory running for 14 successive days, 90 minutes a day at a constant speed of 11 meters per minute. The compulsory wheel was similar to the running wheel unless it was rotated by a motor on which the rats had to run. Its rotation speed was adjustable and the number of turns was recorded by an automated system (4).
2.8. Drug
Morphine sulfate (Iran, Drug Distribution) and sodium chloride 9% (Mashhad, Samen Pharmacy) and normal saline were used in this study.
The entire phases of the experiment procedure are delineated in Figure 1.
2.9. Data Analysis
SPSS- 20 and one- way Analysis of Variance, Tukey test and independent T test were used to analyze the data. Data normality was evaluated using the Shapiro-wilk Test and the difference between the groups was measured considering P < 0.05 as significant. It is important to note that in the course of the experiment we lost one rat in the compulsory exercise group and 4 rats in the morphine group. An animal’s death during the study is out of control and is due to the nature of the intervention. Before doing a statistical analysis we checked the normality of data with Shapiro-Wilk Test. The test results approve its normality, which makes it possible to compare these groups with each other.
3. Results
3.1. Condition Place Preference
Descriptive statistics including mean, standard deviation (SD), and ranges of CPP score in 4 groups are presented in Table 1.
| Groups | Number | Mean ± SD | Minimum | Maximum |
|---|---|---|---|---|
| Compulsory exercise | 7 | 47.43 ± 92.06 | -77 | 164 |
| Voluntary exercise | 8 | 157.88 ± 135.59 | 3 | 442 |
| Morphine | 4 | 239.50 ± 138.49 | 84 | 386 |
| Control | 8 | -17.63 ± 60.80 | -99 | 88 |
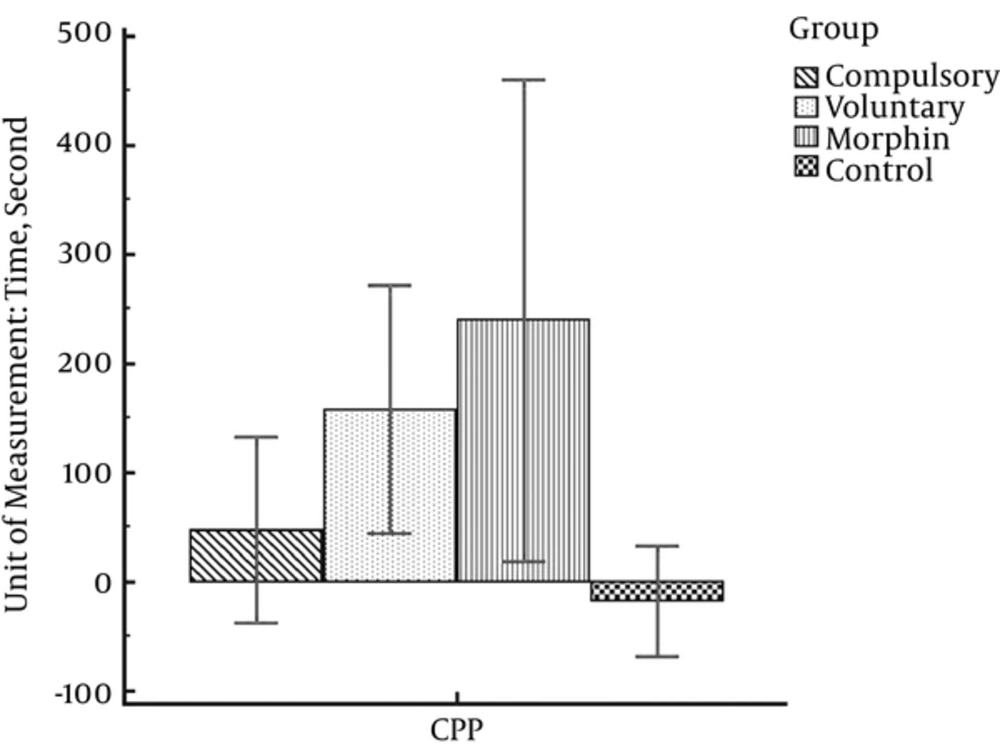
As shown in Table 1, the highest mean score of CPP belongs to the morphine group (M = 239.50; SD = 138.49) and the lowest CPP mean score (M = -17.63; SD = 60.80) is in control group. The mean score (SD) obtained by rats in voluntary and compulsory exercise groups are 157.88 (135.59) and 47.43 (92.06), respectively.
The results of the one-way analysis of variance show that there is a significant difference of CPP between different experimental groups (F (3, 26) = 6.75, P = 0.002). Moreover, the statistical tests showed that morphine injection significantly induced CPP in the morphine and voluntary exercise groups as compared with the control group (saline/saline) (P < 0.01). However, this difference was not significant in the compulsory exercise group. Moreover, the rats in the compulsory exercise group had significantly fewer tendencies towards morphine than the rats in the morphine group (Figure 2).
Considering Figure 2, the highest mean score of CPP is obtained by the morphine group and the lowest score is for the control group. Moreover, the CPP mean score in the voluntary exercise group is less than the morphine group and more than the compulsory exercise group. In general, it shows that morphine dependency is more possible in morphine, voluntary exercise and compulsory exercise, respectively.
3.2. Locomotion Activity
Descriptive statistics of rat’s locomotion activity is presented in Table 2.
| Groups | Number | Mean ± SD | Minimum | Maximum |
|---|---|---|---|---|
| Compulsory exercise | 7 | 48.14 ± 17.12 | 19 | 72 |
| Voluntary exercise | 8 | 63.13 ± 28.75 | 18 | 95 |
| Morphine | 4 | 21.30 ± 21.30 | 26 | 78 |
| Control | 8 | 26.43 ± 26.43 | 4 | 91 |
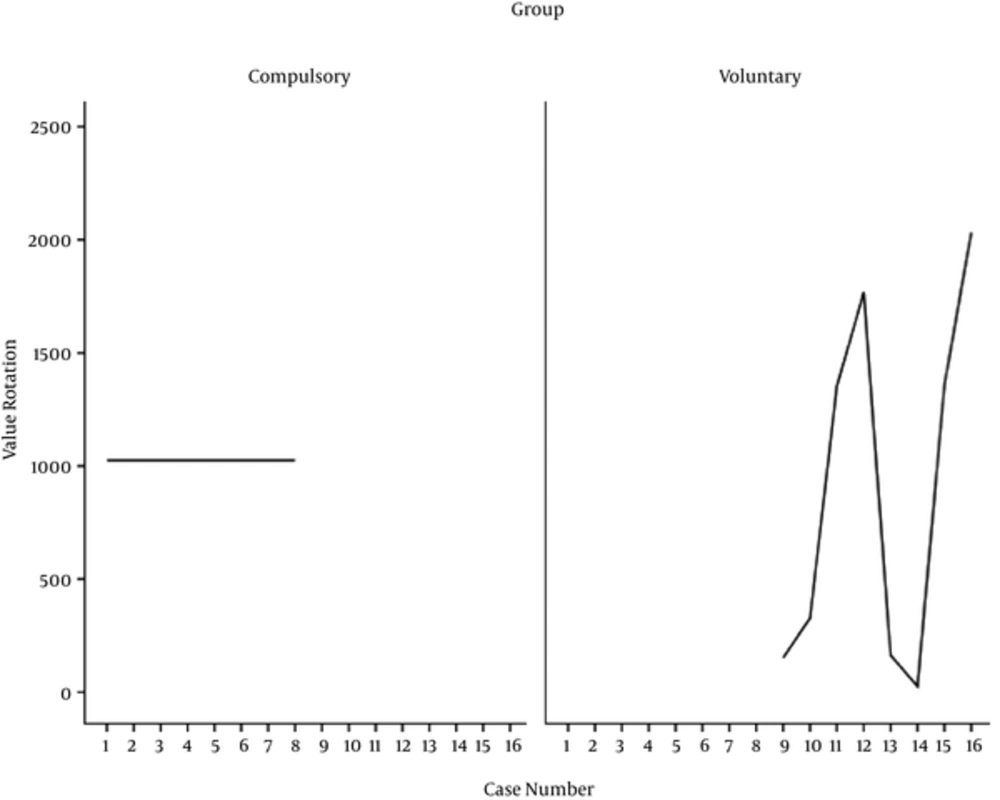
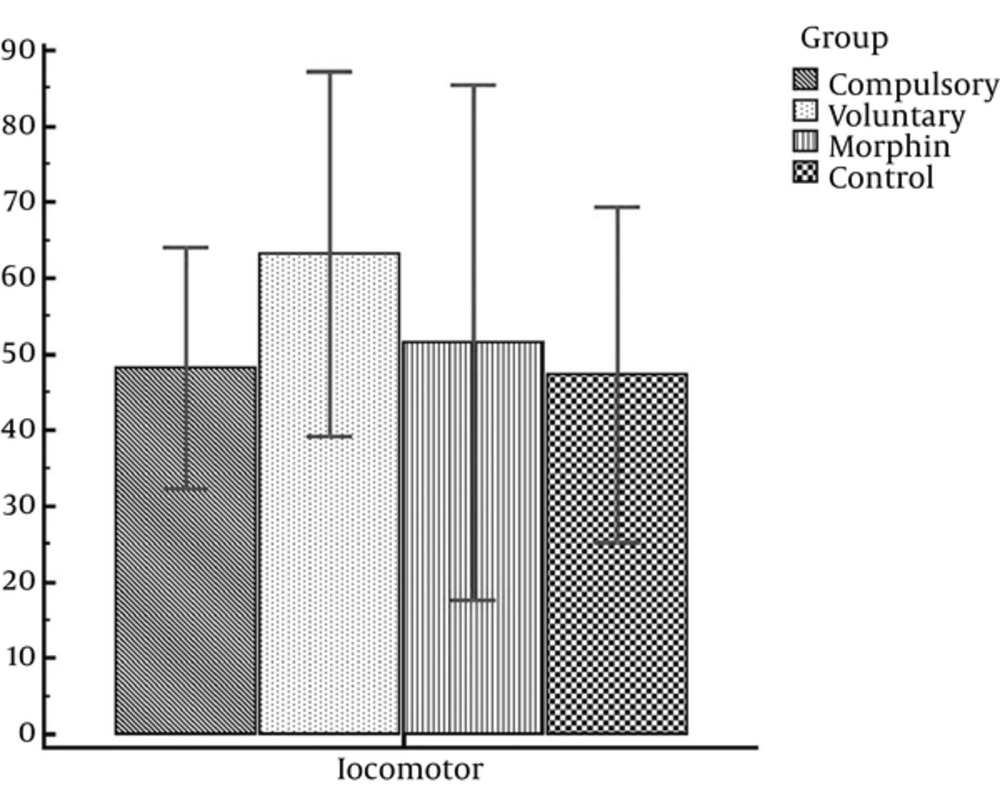
Considering Table 2, the mean score of rat’s locomotion activity in the compulsory, voluntary, morphine, and control groups are calculated to be 48.14, 63.13, 51.50, and 47.25, respectively. In this regard, the number of wheel turns in the voluntary and compulsory groups for 14 days of experiment is reported in Figure 3.
The results of the present study revealed that voluntary active rats tested for CPP averaged 896.32 (SD = 814.32) wheel rotations per day. As can be seen in Figure 3, the maximum rate of wheel rotation is recorded for the rat number 16 and the minimum rate of wheel rotation is recorded for the rat number 14.
Since CPP expression is under effect of drug locomotor side effects, including activity incensement or decrement, we investigated animal’s locomotion activity. Thus, locomotion activity of 3 experimental groups (voluntary, compulsively, and morphine groups) was compared with the control group and the statistical analysis showed no significant difference between the locomotion activity of the control group (saline/saline) and the rats in the compulsory group (t = 0.08; P = 0.94); voluntary group (t = 1.15; P = 0.27); and those rats who received morphine in the conditioning stage (0.28; P = 0.79) (Figure 4).
3.3. Body Weights
Across the study, the mean body weight of full sample in the first, 15th and 19th days of the experiment were 260/00 g (SD = 22.63); 295.38 g (SD = 23.52) and 305.41 g (SD = 26.42), respectively. Below you can find the mean body weight of the rats in the 4 groups separately (Table 3).
| Groups | Days | Number | Mean ± SD | Minimum | Maximum |
|---|---|---|---|---|---|
| Compulsory group | 1st | 8 | 258.75 ± 18.47 | 240 | 285 |
| 15th | 8 | 290.13 ± 17.98 | 265 | 320 | |
| 19th | 7 | 294.43 ± 15.99 | 263 | 310 | |
| Voluntary group | 1st | 8 | 254.88 ± 25.31 | 211 | 285 |
| 15th | 8 | 298.75 ± 19.77 | 275 | 328 | |
| 19th | 8 | 301.00 ± 20.81 | 277 | 342 | |
| Morphine | 1st | 8 | 256.25 ± 24.31 | 220 | 280 |
| 15th | 8 | 296.38 ± 27.04 | 260 | 327 | |
| 19th | 4 | 321.00 ± 18.56 | 303 | 338 | |
| Control | 1st | 8 | 270.13 ± 23.02 | 220 | 290 |
| 15th | 8 | 296.25 ± 30.95 | 241 | 331 | |
| 19th | 8 | 311.63 ± 38.31 | 243 | 348 |
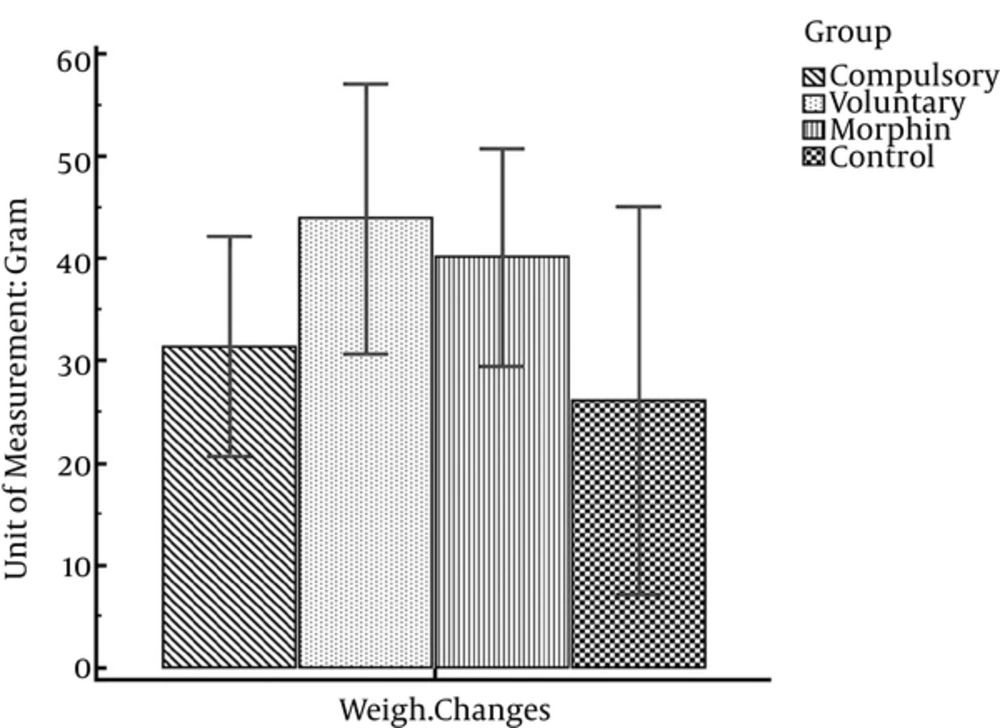
Data presented in Table 3 illustrates that all rats gained weight through the experiment process. So that in all four groups, the maximum mean body weight is on 19th day of the experiment. However, one-way analysis of variance indicates no significant difference of weight changes within the 4 experimental groups (F (3, 26) = 0.68; P = 0.57) (Figure 5).
4. Discussion
The current study was undertaken to test the hypothesis that voluntary and compulsory exercise have a significant difference on morphine CPP in male rats. Consistently with existing literature (39), the morphine-induced psychological dependence in male Sprague Dawley rats of the voluntary exercise and morphine groups was generally supported, while morphine injection induced no significant psychological dependence in the rats of the compulsory exercise group. Our results also reveal that the rats’ tendency toward morphine in compulsory and voluntary exercise groups was lower than those in the morphine group and this difference was significant in the compulsory exercise group and insignificant in the voluntary exercise group. Besides, the morphine tendency in rats designated to the compulsory exercise group is lower than those in the voluntary group. In other words, compulsive exercise in this range could significantly reduce psychological dependence to morphine while this reduction was not significant for voluntary exercise in this experiment.
Another hypothesis, which was tested in this experiment, was an insignificant difference between weight changes and locomotion activity of rats in 4 experimental groups (compulsory, voluntary, morphine, and control groups). In this regard, the results reported in this experiment indicate that weight change difference in different experimental groups is not significant. Our findings are also in agreement with the recent studies providing evidence that peritoneal morphine injection in compulsive exercise, voluntary exercise, and morphine groups, compared to the control group, induced no significant change in locomotor activity. Thus, conditioned stimuli are the main determinant in locomotor response to morphine (40).
Emerging evidence suggests that to observe the effect of voluntary exercise, the animals must run at least a hundred meters in 24 hours (31). In the current study the number of rat’s rotation in voluntary group was more than baseline and provided satisfactory running measures. Furthermore, all rats in the compulsory exercise group ran with the same speed in the same time limit (90 minutes per day, with the speed of 11 meters per minute). In sum, the rats in the compulsory exercise ran 1,025 meters in the experiment process, which is acceptable.
With regards to the clinical observations during the experiment, it seems that there may be a significant difference between voluntary exercise and the morphine groups with respect to morphine tendency as well as between compulsory and voluntary groups in cases where the volume of samples increases. However, we couldn’t find any related literature regarding the comparison of voluntary and compulsory exercise effects on morphine CPP, similar previous researches demonstrate that voluntary and compulsory exercises are effective in decreasing physical dependency and increasing learning and memory (27, 41, 42). It is important to note that compulsory exercise, due to the associated high rate of stress, may not be as effective as voluntary exercise. However, in the current study, inconsistent with the previous studies, compulsory exercise was more effective in reducing rats morphine tendency and dependency.
To clarify the CPP procedure, much of the research to date has focused on dopamine neurotransmitter. It is suggested that morphine consumption leads to the suppression of mu opioid receptors in the ventral tegmental area by reducing cyclic adenosine monophosphate. In this way, suppression is removed from dopaminergic neurons of the ventral tegmental area and dopamine release increases in nucleus accumbens (43). We posit that morphine induces CPP by increasing dopamine in nucleus accumbens (32). Supported by the literature, the present study provided an argument to support that intra peritoneal morphine injection caused no significant difference in the locomotion activity as compared with the control group. Therefore, conditioned stimulants are the essential determiner in the locomotor response to morphine (40).
Moreover, there are some evidences suggesting that both types of exercise (treadmill and voluntary) attenuate self-administration of cocaine (33), morphine (41), amphetamine (44), and alcohol (45) in laboratory rodents. These results support the hypothesis that exercise is an effective intervention in ensuring drug abuse prevention and treatment programs. Some studies have reported that the elevated amount of endorphin is directly associated with exercise intensity while other studies provided no evidence for this association (23).
Previous findings have suggested that short-term compulsory exercise can increase dependence symptoms after naloxone injection and decrease dependence symptoms in addicted animals (4). The results of this study indicated that morphine tendency in rats of the compulsory exercise group was significantly less than those of the morphine group. No significant difference was found between the compulsory and voluntary exercise groups with regards to morphine tendency (P < 0.05). In other words, compulsory exercise can significantly reduce psychological dependence to morphine but this difference was not significant in the voluntary exercise.
Given the clinical observations and the amount of rats’ death after morphine injection in the conditioning stage, the rats’ physical resistance against morphine injection dose in the voluntary exercise group was more than that of the compulsory exercise group. The rats’ physical resistance against the morphine injection dose was also more in the compulsory exercise group as compared with that of the morphine group. Despite these promising findings, some limitations to the study should be acknowledged. First, our sample was composed of only male rats with the least possible number of rats due to ethical considerations. Second, during the experiment, morphine administration to rats resulted in both rapid and delayed deaths in 6 rats. These elements may influence our results. Further studies should include a bigger sample including female rats, because female rats are more likely to run. It is suggested that future studies with a closer outlook at this field, try to conduct the same experiments at night, since rats are more active at night and there is less danger. In general, our findings suggest that exercise reduces psychological dependence to morphine, thus it is an appropriate procedure for addiction prevention and also complete other treatment programs as a natural reward system.