1. Background
Absence seizure is a kind of epilepsy disease accompanied by temporary loss of consciousness. The spike-wave potentiation (SWP), which appeared spontaneously and synchronously is the top specs of the electro-encephalogram (EEG) in the absence of epilepsy (1). WAG/Rij (Wistar Albino Glaxo Rijswijk) rat, which is a verified genetic model of absence seizure, is commonly used for the study of the pathophysiology of absence seizure. An age-dependent progression of absence seizures has been reported in WAG/Rij rats (2). The post-natal progression of SWPs in WAG/Rij rats can be triggered by cumulative structural and functional changes in the brain cells and neuronal networks, including changes in the excitability of individual neurons, the strength of network synaptic activity, and/or alteration in inhibitory interneurons, to generate synchronized discharges. Abnormal interconnection in different areas of the cortico-thalamocortical network has an important role in the appearance of SWPs (3). In addition, the tune of thalamocortical rhythmicity depends on the distribution of the cortical receptors (4-6).
The MET gene encodes the tyrosine kinase receptor known as c-Met. HGF is a major known ligand of the MET receptor. C-Met receptors have emerged as transmission regulators of GABA and dopamine neurotransmitters, and as potent modulators of glutamate-related synaptic plasticity (7). These receptors have incredible roles in creating a balance between excitatory and inhibitory neurotransmission, normal synaptic plasticity, and sensorimotor gating (8). The retention of excitation/inhibition balance is serious for the homeostatic control of brain function, and its impairment can result in the development of several brain disorders such as epilepsy (9). C-Met receptors regulate the activity of hippocampal and cortical pyramidal neurons, while dysfunction of c-Met channels perturb the neuronal network activity, functional connectivity, and synaptic plasticity (10, 11).
2. Objectives
Therefore, the present study aimed to investigate the alteration of c-Met receptors in both hippocampal and cortical brain areas during the absence seizures appearance in the WAG/Rij rats, which was known as the most valid laboratory rat model of absence attacks. It should be noted that absence attacks appear in the adult WAG/Rij rats spontaneously, approximately after three months of age.
3. Methods
3.1. Animals
Male Wistar and WAG/Rij rats were included in the study and adapted under standard laboratory conditions of 22ºC environmental temperature, 40% dampness, and 12 hours of light and dark with unrestricted access to food and drink. Animals were divided into four groups (n = 12) of two- and six-month-old WAG/Rij and Wistar rats. Animal trials were performed based on the guidelines offered by the Ethical Committee of Iran University of Medical Sciences, Tehran, Iran, and in accordance with Ethics in Animal Experiments (IR.IUMS.RFC.1396.30641).
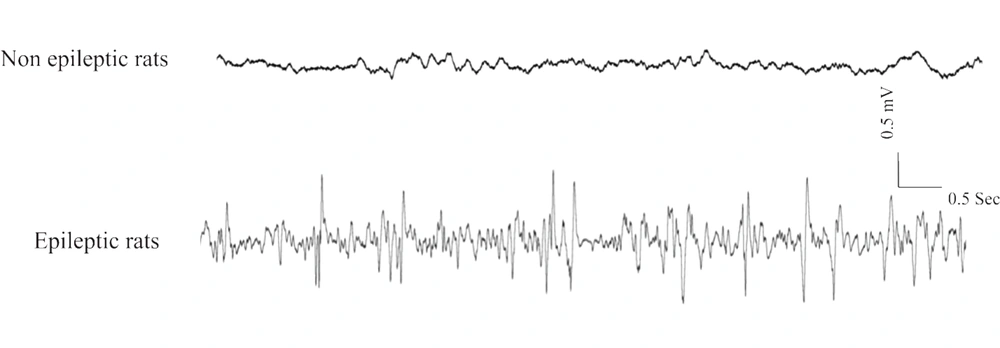
To determine epileptic rats, ECoG recording (electrocorticogram) was carried out for at least six hours under sedated condition by intraperitoneal fentanyl injection (3 μg/kg) every 20 - 30 minutes (12). Epileptic WAG/Rij rats showing SWP in ECoG were included in the epileptic group (5). Two-month-old WAG/Rij rats as well as two- and six-month-old Wistar rats not exhibiting SWP in ECoG, were selected as the non-epileptic rats (Figure 1) (13).
Monitoring ECoG in the experimental groups. The ECoG was recorded by implemented electrodes on the somatosensory cortex. Up-trace indicates ECoG was recorded from non-epileptic rats (two and six-monhs old Wistar and also two-month-old WAG/Rij rats). Lower-trace indicates ECoG was recorded from epileptic rats (six-month-old WAG/Rij rats).
3.2. Molecular Study
3.2.1. RNA Extraction and Reverse Transcription
After inducing deep anesthesia by using chloralhydrate and transcardially perfusion with 200 mL of saline, the animals were decapitated, and the somatosensory cortex and hippocampus were dissected. Total tissue RNA was extracted from brain samples using Gene ALL Ribospin Kit.
A DNase treatment was performed using (RNase-Free DNase Set "DNase I," Qiagen) to remove genomic DNA. The Maxima first strand cDNA synthesis kit (Thermo Scientific) was used to synthesize complementary DNA (cDNA) from all of the recovered RNA (500 ng) based on the manufacturer's conductions.
3.2.2. Real-Time PCR
MET and β-actin primers sequences were gained from prior works (12, 14). Real-time PCR reactions were carried out on a CFX 96 Real-Time System (Bio-Rad). All data were analyzed by duplicated samples. Amplified products were detected by Eva Green dye. The primer sequences for internal control and the MET gene are as shown in Table 1. Conditions for PCR were an initial denaturation at 95°C (15 min), 40 denaturation cycles at 95°C (30 sec), primer annealing at 72°C (45 sec), and elongation at 72°C (30 sec). To assess product specificity, a melting curve analysis was performed following RT-PCR.
| Gene | Forward Primer | Reverse Primer | Length |
|---|---|---|---|
| MET | 5'-TCCTGACGGCAAGGATGAC3' | 5'TGATGATACCCACATTGGTGTTC3' | 131 bp |
| β-Actin | 5'AAGTCCCTCACCCTCCCAAAAG3' | 5'AAGCAATGCTGTCACCTTCCC3’ | 98 bp |
3.2.3. Western Blot Analysis
The somatosensory cortex and hippocampus samples homogenization was performed using lysis buffer including: tris buffer (50 mM), EDTA (1 mM), 1% Triton, phenyl-Methyl-sulfonyl fluoride (1 mM), aprotinin (1 µg/mL), pepstatin (1 µg/mL), and leupeptin (1 µg/mL) in 4ºC. Protein extracts were normalized for concentration using the Bradford test. Then 0.5µg/µL of total cell protein per sample in the buffer of SDS-PAGE (0.25 M Tris-HCl, 10% (v/v) glycerol, 10% (wt/vol) SDS, and 10 mM dithiothreitol) at pH 6.8 were diluted. Protein samples were electrophoresed on a 12% SDS-polyacrylamide gel, transferred to PVDF membranes, and blocked with 5% non-fat dried milk in TBST buffer (Tween-200, 1%; Tris-HCl, 100 mM; NaCl, 150 mM, pH 7.4) for the night. Then the blots were incubated for three hours with mouse anti-MET mAb (Abcam) and mouse anti-β actin mAb (Sigma) at room temperature. After washing for three times (15 min each time), filters were incubated with secondary antibodies for 90 min. The secondary antibody was HRP-conjugated goat anti-mouse; Santa Cruz. ECL visualized the immuno-reactivity (Amersham Biosciences, Freiburg, Germany). The blots for 5 - 30 sec were exposed to sensitive X-ray film, and then films were scanned on a Bio-Rad scanner for densitometry. Quantitative analysis was performed by the monomeric band's data using Image J software.
3.3. Immunohistochemistry
After inducing deep anesthesia (chloral hydrate, i.p. injection) and transcardial perfusion by using 250 mL of normal saline and 400 mL of PFA (paraformaldehyde) 4% fixative solution, the brains were isolated and inserted in PFA 4% for about five days at 4ºC. After fixation and process steps for immunohistochemistry (IHC) studies, the serial coronal sections in 8μm thickness were prepared. Three slices from each brain were selected and rehydrated by the series of graduated alcohol, washed three times by PBS (pH = 7.4), and incubated for 5 min in the blockade solution (H2O2/ methanol, 3 %). Sections were boiled in citrate buffer at 95°C (pH = 6.0, 10 min) and kept at room temperature to cool. The sections were incubated in the normal goat serum (10%) and X-100 solution of Triton (0.2%) for 1 hour. Sections were incubated at 4ºC with the primary antibody against c-Met (1: 300) overnight, washed three times with PBS, and incubated by secondary antibody (goat anti-rabbit which conjugated with horseradish peroxidase (RNASEN; Abcam). The antibody was diluted in a ratio of 1: 100 in PBS with Triton X-100 0.3% and NGS 5% at 22ºC for 1 hour. To visualize the reactions, slides were incubated in 0.5 µL DAB (three-3'diaminobenzidine) and the buffer of peroxide (1.5 µL, 5 - 10 min), and were dyed with hematoxylin.
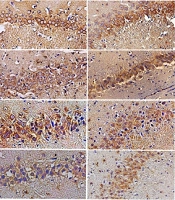
Negative control was carried out by removing the primary antibody incubation. Images were acquired using a digital camera (Nikon, objective lens 40X) linked to a microscope. The c-Met antibody labeled cells were counted in 1 mm2 in the areas of the somatosensory cortex (i.e., external and internal granular layers) and the hippocampus (CA1 and CA3).
3.4. Statistical Analysis
The Mean ± SEM of the data were analysed statistically using the one-way ANOVA followed by Bonferroni post-hoc test in the SPSS Statistics 22. The P < 0.05 was considered as the significant level.
4. Results
4.1. mRNA Level of c-Met Receptors
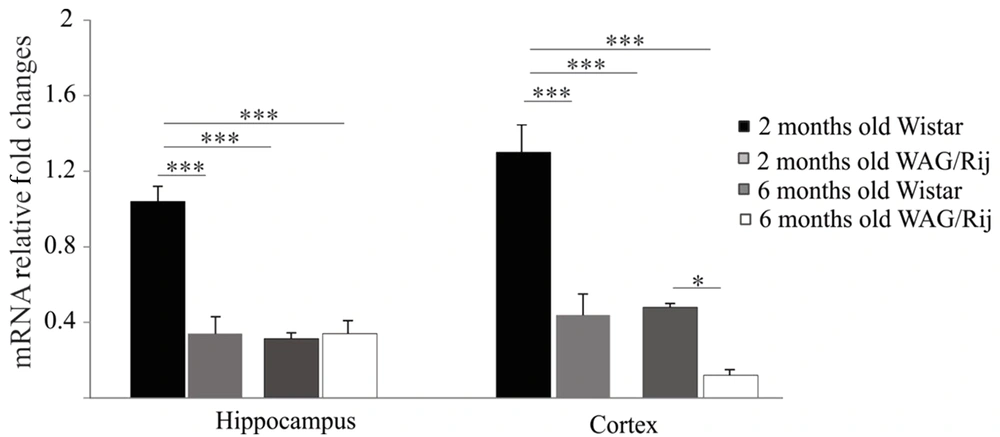
First, the expression of the MET gene in the hippocampus and somatosensory cortex of the two- and six-month-old WAG/Rij and Wistar rats was examined using RT-PCR.
A significantly high mRNA expression of c-Met was observed in the hippocampus of the two-month-old Wistar rats compared to that in the hippocampus of the six-month-old Wistar rats (P < 0.001). The results also indicated that the MET gene expression was lower in two-month-old WAG/Rij rats in comparison to the same-age Wistar group (P < 0.001; Figure 2).
MET mRNA levels were considerably higher in the cortex of the two-month-old Wistar rats compared to those in the cortexes of two- and six-month-old WAG/Rij rats (P < 0.01). Likewise, the gene expression of MET was significantly decreased in six-month-old WAG/Rij rats in comparison with that in the same-age of Wistar group (P < 0.01).
4.2. Protein Level of c-Met Receptors
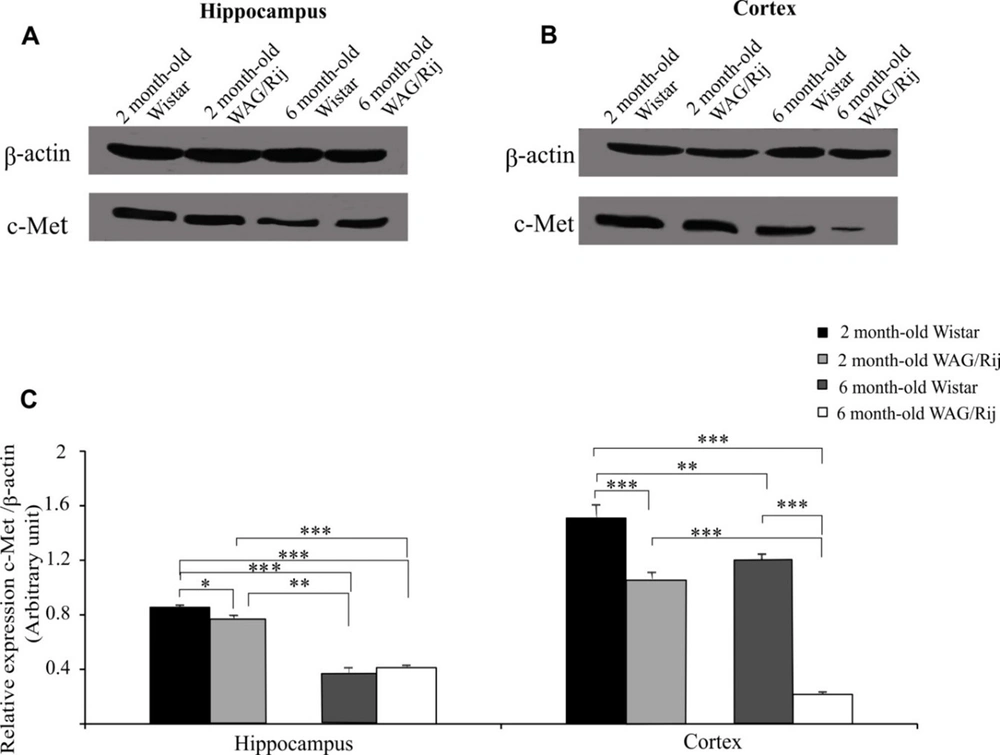
Next, the hippocampal and cortical c-Met receptor protein expression levels were determined using a western blot (Figure 3A and B).
Western blot analysis of c-Met protein in the cortex and hippocampus. A and B, Immunoblotting images of hippocampal and cortical c-Met protein in two- and six-month-old Wistar and WAG/Rij (epileptic) rats; C, The quantitative results of hippocampal and cortical c-Met protein expression. The symbols of *, ** and *** indicated P < 0.05, P < 0.01 and P < 0.001 (data has been illustrated as the mean ± S.E.M).
As for the hippocampus, the six-month-old Wistar and WAG/Rij rats displayed significantly low-level c-Met protein expression compared with the two-month-old Wistar and WAG/Rij rats (P < 0.001; Figure 3C). Interestingly, c-Met protein expression in two-month-old WAG/Rij rats was lower than that in the same-age Wistar rats (P < 0.05); however, no significant difference was observed between the six-month-old Wistar and WAG/Rij rats in this regard (Figure 3C).
As for the cortex, the two-month-old WAG/Rij rats displayed a lower level of c-Met protein expression than the age-matched Wistar rats (P < 0.001). C-Met protein expression was reduced in six-month-old Wistar rats compared to that in two-month-old Wistar rats (P < 0.01). In addition, our results revealed that there was a significant decline in the expression of the c-Met protein in six-month-old WAG/Rij rats compared to that in two-month-old WAG/Rij rats and six-month-old Wistar rats (P < 0.001 and P < 0.001 respectively; Figure 3C).
4.3. Distribution of c-Met Receptors
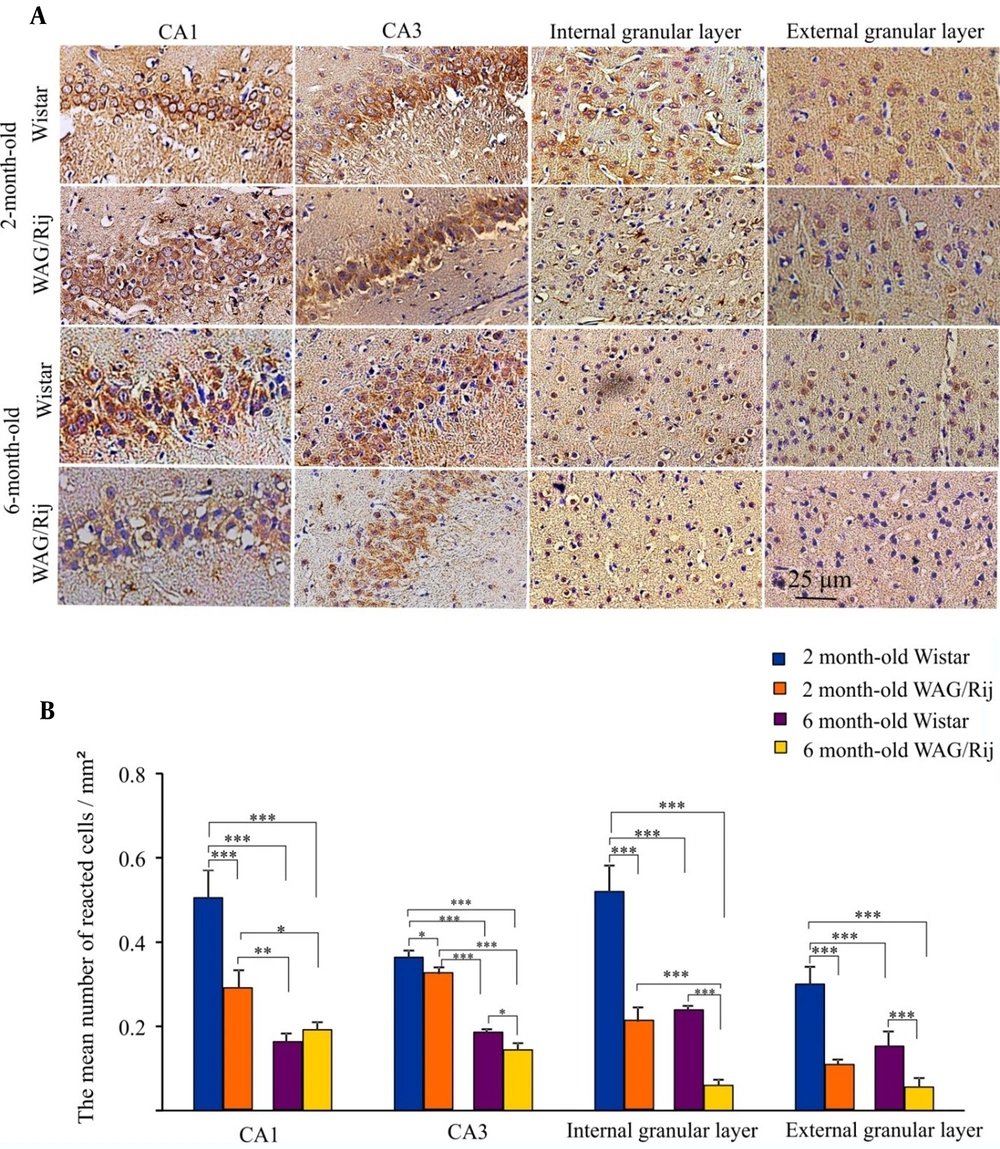
The mean number of immunohistochemistry spots was analyzed in the CA1 and CA3 hippocampal regions as well as in the somatosensory cortex (Figure 4A and B).
Immunohistochemistry results of cortical and hippocampal c-Met receptor distribution. A, Photomicrographs of c-Met-labeled cells (magnification: 200X); B, The bar graph shows the quantitative results of c-Met-labeled cells. Values have been indicated as means ± SEM (the symbols of *, ** and *** indicated P < 0.05, P < 0.01 and P < 0.001).
As for the CA1 area, the expression of c-Met receptors in the two- and six-month-old WAG/Rij rats, as well as in the six-month-old Wistar rats, was significantly lower than that in two-month-old Wistar rats (P < 0.001). The c-Met distribution reduced significantly in the six-month-old Wistar (P < 0.01) and WAG/Rij rats (P < 0.05) compared to the two-month-old WAG/Rij rats. The mean number of reacted cells/mm2 was 0.5 ± 0.025 and 0.28 ± 0.02 in two-month-old Wistar and WAG/Rij rats, and it was 0.16 ± 0.01 and 0.19 ± 0.01 in six-month-old Wistar and WAG/Rij rats, respectively.
As for the CA3 area, the expression of c-Met receptors in the two-month-old WAG/Rij rats was significantly lower than that in the two-month-old Wistar rats (P < 0.05). The c-Met distribution remarkably reduced in the six-month-old Wistar and WAG/Rij rats in comparison to the two-month-old WAG/Rij rats (P < 0.001). Furthermore, the c-Met receptors were significantly less-expressed in the CA3 area of six-month-old WAG/Rij rats compared to the same-age Wistar rats (P < 0.05). The mean number of reacted cells/mm2 was 0.36 ± 0.008 and 0.32 ± 0.009 in the two-month-old Wistar and WAG/Rij rats, and it was 0.18 ± 0.005 and 0.01 ± 0.009 in the six-month-old Wistar and WAG/Rij rats, respectively.
The expression of c-Met receptors in the internal granular layer of the cortex of the two- and six-month-old WAG/Rij rats and six-month-old Wistar rats was significantly lower than that in the internal granular layer of the cortex of the two-month-old Wistar group (P < 0.001). In addition, the c-Met receptors were significantly less-expressed in six-month-old WAG/Rij rats compared to the group of six-month-old Wistar and two-month-old WAG/Rij (P < 0.001). The mean number of reacted cells/mm2 was 0.52 ± 0.03 and 0.21 ± 0.01 in the two-month-old Wistar and WAG/Rij rats, and it was 0.23 ± 0.005 and 0.059 ± 0.006 in the six-month-old Wistar and WAG/Rij rats, respectively.
The expression of c-Met receptors in the external granular layer of the cortex of the two- and six-month-old WAG/Rij rats as well as the six-month-old Wistar rats, was significantly lower than that in the external granular layer of the cortex of the two-month-old Wistar rats (P < 0.001). The c-Met receptors reduction was also significant in the six-month-old WAG/Rij rats compared to six-month-old Wistar rats (P < 0.001). The mean number of reacted cells/mm2 was 0.30 ± 0.02 and 0.10 ± 0.007 in two-month-old Wistar and WAG/Rij rats, and it was 0.15 ± 0.01 and 0.055 ± 0.01 in six-month-old Wistar and WAG/Rij rats, respectively.
5. Discussion
Our study findings revealed that the gene and protein expressions of both hippocampal and cortical c-Met receptors were significantly lower in WAG/Rij rats compared to Wistar rats. Our findings also showed that the gene expression and protein level of the c-Met receptor in the epileptic rats decreased as their age increased. The WAG/Rij rat is a naturally occurring genetic model that is widely used for absence epilepsy investigations (15). Electrophysiological records and behavioural tests clearly show absent seizures in each member of WAG/Rij rats. SWPs begin to appear on the cortical records of the two-month-old WAG/Rij rats, and their duration and frequency increase with age (16). The somatosensory cortex has an important role in triggering and generating of SWPs (17). The somatosensory cortex has typically six layers. The most superficial of them is layer one (next to the brain surface), and the deepest is layer six. The sensory signals first go into the neural layer four and then spread into more superficial and deeper layers of the cortex. The processed signals are dispatched to the thalamus from the deepest layer (i.e., layer six). These signals are involved in set-out the excitation of thalamic nuclei (12, 18).
Electrocorticographic (ECoG) analysis of the WAG/Rij rats has indicated that SWPs are initiated in the underside layers of the primary neocortex and, from there quickly spread to motor cortices and thalamic nuclei (19, 20). Deep layers of the somatosensory cortex display ictogenic properties known to have depolarized membrane potential leading to hyperexcitability, fast activation, and hyper synchronization in the absence seizures (4, 21). During SWPs, moreover, enhanced synchronization and functional connectivity between the hippocampus and thalamocortical network have been reported in WAG/Rij rats. The hippocampus is involved in absence seizures through thalamocortical activity (22-24).
Previous studies in WAG/Rij rats reported progressive changes in the duration and frequency of SWPs in adult animals (25), associated with alteration in the density of different ligand and voltage-gated ion channels in the somatosensory cortex (26-28).
The c-Met is a tyrosine kinase transmembrane receptor, and HGF (hepatocyte growth factor) is its known ligand. These potent signaling molecules have key roles in many neurodevelopmental and neuroplasticity aspects. C-Met receptors are distributed in several brain regions including neurons of the olfactory bulb, cerebral cortex, hippocampus, amygdala, and cerebellum (29). The extensive expression of c-Met receptors has been found in inhibitory interneurons of the cortex (30). The critical role of c-Met receptors in cortical neural circuit formation and brain development has also been reported. C-Met receptors help radial glia aliveness and normal migration of neurons in the cerebral cortex and cerebellum during brain development (7). These receptors participate in regulating the direction of movement of GABAergic interneurons from the originating sites (the ganglionic eminence) to the neocortex, where they then differentiate and function (31). Impaired migration of inhibitory interneurons from the ganglionic eminences to the neocortex leads to hyperexcitability and a tendency to seizures (32).
Comprehension of the role of c-Met receptors in the development of circuits or synaptic function might have a critical role in understanding the etiology of epilepsy. HGF and c-Met promote neurite outgrowth and dendritic maturation during the differentiation of hippocampal neurons, as well as stimulate dendrite arborization, regulate spines size in mature neurons, and guide axons to their targets (33-35). Moreover, the role of c-Met receptors in the regulation of LTP (long-term potentiation) and increment of synaptic transmission of the entorhinal-hippocampal track has been documented (36). Therefore, alteration in c-Met receptor expression can impair synaptic plasticity and increase the susceptibility to seizures, as our results showed that c-Met receptor expression was lower in epileptic rats compared to Wistar rats.
More importantly, a disbalance in the release of glutamatergic excitatory and GABAergic inhibitory neurotransmitters is a chief etiology of absence seizure. An important role of c-Met receptors is to balance the excitatory and inhibitory neurotransmission in the cortex (8, 37). The cortex and hippocampus GABAergic interneurons express MET mRNAs (29, 38). These receptors regulate cellular properties associated with GABAergic interneurons (8). We observed that mutation in the activator of urokinase plasminogen receptor, an important agent for activation of HGF, decreased the expression of HGF, that was associated with a noticeable reduction in cortical GABAergic interneurons, the development of spontaneous seizures, and also increased sensitivity to pharmacologically induced seizures (39, 40).
According to our findings, c-Met gene and protein expression, as well as protein distribution, were lower in the two-month-old WAG/Rij rats compared to the same-age Wistar rats, and in six-month-old WAG/Rij rats compared to two-month-old ones. Previous studies showed that a decrease in c-Met activity during the development of WAG/Rij rats may have led to a deficiency in the inhibitory inputs of cortical neurons followed by a reduction in the release of GABA by cortical interneurons. Developmental reduction in the cortical c-Met signaling may have played a role in the appearance of SWP in the WAG/Rij rats.
Involvement of the hippocampus in absence seizures may contribute to cognitive impairment of patients with absence seizures (23). Cognitive dysfunctions (e.g., memory and attention impairment) due to alterations in signaling pathways and neuronal network dysfunction are common comorbidities that occur in epilepsy (41). Cognitive, emotional, and behavioral abnormalities have been reported in children with absence epilepsy (42, 43). Our previous studies documented the cognitive and memory impairments in epileptic WAG/Rij rats (5, 13). Seemingly, the reduced c-Met activity plays a role in memory and cognitive deficit in WAG/Rij rats. It has been shown that c-Met is capable of improving cognitive impairment in aged mice (44). The administration of HGF, the known ligand of the c-Met receptors, to kainate-induced epileptic model rats has been found to alleviate the seizure scores and improve learning and memory (45). Moreover, angiotensin IV (Ang IV) has been indicated to have anticonvulsant activity in seizure models (46). The beneficial effects of Ang IV on synaptic plasticity, memory improvement, and alleviation of seizures in animal models of epilepsy and cognitive deficits convinced the researchers to find a mechanism for these effects; therefore, several studies were conducted, and the results revealed that the biological actions of Ang IV were related to interaction with HGF and subsequently regulation of c-Met activity (44). c-MET is a high-affinity binding site mediating the biological effects of angiotensin IV (47).
In sum, our study results demonstrated that the gene and protein expression as well as the protein distribution of the c-Met receptor in the somatosensory cortex and hippocampus were inversely associated with the development of epileptic seizures. The above-mentioned studies partially explain how activation of c-MET may account for its anticonvulsive and anti-epileptogenic effects. Clarifying these mechanisms may have effectively contributed to the establishment of novel strategies in the treatment of epilepsy.