1. Background
Multiple sclerosis (MS) is the most common autoimmune inflammatory disorder of the central nervous system and is caused by immune dysregulation triggered by genetic and environmental factors, leading to demyelination and axonal loss. The disease usually presents clinically between the ages of 15 and 45 years, mainly affects young women, and is one of the causes of disability in young adults (1-3). Multiple sclerosis pathogenesis is an immune system attack against central nervous system antigens activated by CD4 cells and the possible involvement of B lymphocytes (4, 5). Immunopathogenesis of MS appears to be due to loss of resistance to local antigens in the central nervous system, especially myelin, which leads to permanent activation of peripheral T lymphocytes (1, 6, 7). Myelin-activated T lymphocytes can cross the blood-brain barrier, a process that results from the interaction between very late antigen 4 (VLA-4) at the level of T lymphocytes and vascular cell adhesion molecule-1 (VCAM-1) in vascular endothelial cells (8, 9).
MS has subtypes that differ in prognosis and type of therapeutic intervention (10, 11). At the time of diagnosis, 90% of patients will have a relapsing-remitting disease course. However, almost all neurological symptoms appear later in the disease and often progress to physical and cognitive disabilities (12, 13). Therefore, studying the etiology and factors affecting this disease can reduce its prevalence and, consequently, the psychological and economic burden on society (13). The etiology of MS remains unknown; however, it is believed that autoimmune mechanisms influenced by genetic and environmental factors, such as infectious causes, are involved (14). Chlamydia pneumoniae, HHV6, EBV, coronavirus, VZV, and Helicobacter pylori are known infectious agents (15-19). According to the global classification, Iran is one of the regions with a low prevalence of MS; however, studies show an increasing prevalence in recent years (20). This rate is reported to be 21.6 per 100,000 people in Khorasan Razavi province (21).
Helicobacter pylori is a gram-negative bacterium isolated from the gastric epithelium's luminal surface and chronically infects more than 50% of the population. Infection with these bacteria increases the risk of stomach diseases, such as ulcers (nearly in duodenal or gastric of 1 – 10% of infected patients), gastric cancer (in 0.1 to 3%), and gastric mucosa-associated lymphoid tissue (MALT) lymphoma (< 0.01%). This infection is more prevalent in Iran than in developed countries or other countries at the same latitude. This can be related to lower socioeconomic status, weaker economic indicators, and living with an extended family in Iran (22). The association between infection with this bacterium and MS is controversial, and no extensive studies have been conducted (23).
2. Objectives
We decided to investigate the relationship between this disease and H. pylori infection, which may assist researchers in finding new theories. Few studies have been conducted in Iran, especially in the east of the country. Evaluation of the serological status of H. pylori in patients with MS and comparison with the control group can be used to determine the possible association between this infection and MS. Finding a relation between H. pylori infection and the prevalence, progression, and the clinical course of the disease can help reduce the incidence and progression of the disease.
3. Methods
3.1. Study Samples
The present study is a descriptive-analytical case-control study. All experiments related to this research were performed in the Antimicrobial Resistance Research Center at Buali Research Institute, Mashhad University of Medical Sciences, in 2017. This study used serum samples from 38 patients with multiple sclerosis referred to the Neurology Clinic of Ghaem Hospital as the study group. A neurologist had previously diagnosed MS according to the McDonald criteria. Forty-one patients admitted to other wards of Ghaem Hospital who did not have symptoms of multiple sclerosis were randomly selected and included in the study as the control group. A questionnaire detailing the onset, progression, and demographics of MS patients was filled out.
Inclusion criteria: All patients referred to the neurology clinic during enrollment were diagnosed with MS and gave written consent to participate in the study.
3.2. Exclusion Criteria
History or presence of any malignancy, known immunodeficiency condition, or mental or neurological condition.
3.3. Sample Collection and Testing
The participants completed a questionnaire regarding the onset and progression of their MS illness. Then, approximately 5 cc of blood was taken from each individual, and after isolation, the serum was kept at -70 temperature until the tests were performed. Serum samples were assessed by a commercial kit (Padtan Elm Company (Iran)) in terms of IgA, IgM, and IgG antibodies against H. pylori using the ELISA method.
3.4. Statistical Analysis
Student's t-test was utilized in the information examination. All information was presented as mean ± standard deviation (SD), and the means, medians, and frequencies were computed as part of the descriptive analysis. We performed both univariate and multivariate studies to evaluate each variable. Nominal variables were compared using the Fisher exact test, while continuous variables were compared using the t-test. P-values less than 0.05 were determined statistically significant. Version 20.0 of SPSS Statistics for Windows was used for data analysis.
4. Results
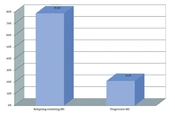
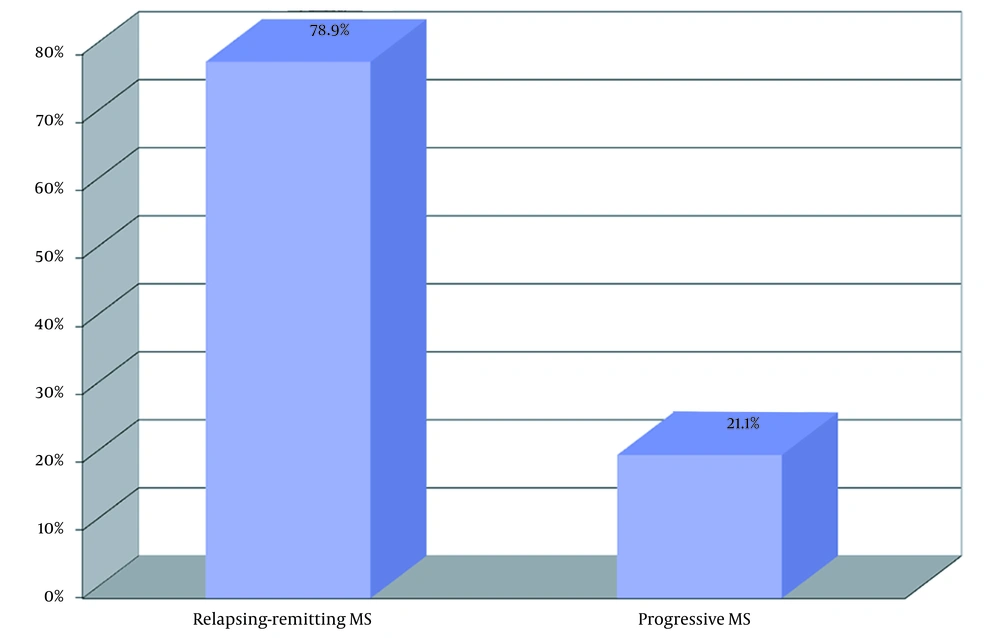
A total of 79 patients participated in this study. Of these, 38 patients had MS, and 41 were healthy individuals. Furthermore, 23 patients were male, and 56 were female, aged 16 - 52. The mean age of participants was compared in the case and the control group and had no statistical differences (P = 0.265). The frequency distribution of women and men in the two groups was similar, and there was no statistically significant difference (P = 0.306). Also, the frequency distribution of jobs in the two groups did not differ significantly (P = 0.250). In MS patients, the age of onset of the disease ranged from 14 to 44 years, with a mean of 28.95 years. Patients were separated into 2 groups regarding MS types: Relapsing-remitting MS (RRMS) and progressive (Figure 1).
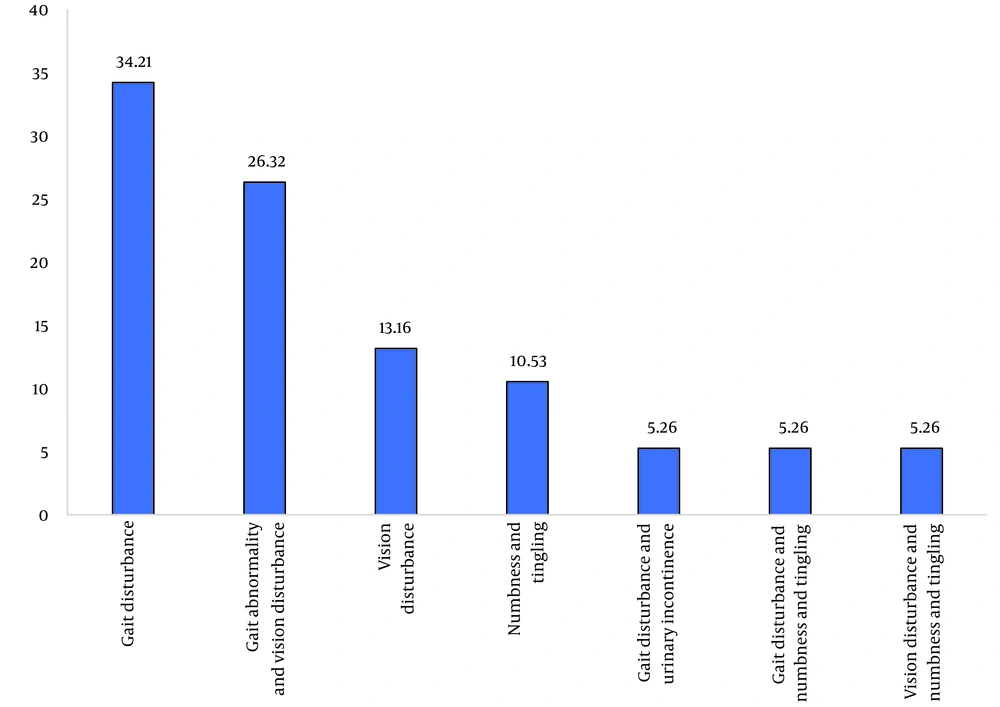
Also, patients were divided into different groups concerning different symptoms (Figure 2). In the MS group, 26 patients (68.4%), and in the control group, 16 patients (39.0%) had anti-H. pylori IgG antibodies. The difference between the two groups was statistically significant (P = 0.009). IgA and IgM antibodies against H. pylori were negative in all samples. The mean age of individuals with antibodies was higher than those without, but this difference was not statistically significant (P = 0.113). The difference between the patients' positive and negative for IgG antibodies regarding sex and MS type is shown in Table 1.
| Variables | Helicobacter pylori IgG | P Value | |
|---|---|---|---|
| Yes | No | ||
| Sex | 0.109 | ||
| Male | |||
| Count | 9 | 14 | |
| % Within sex | 39.1 | 60.9 | |
| % Within IgG H. pylori | 21.4 | 37.8 | |
| Female | |||
| Count | 33 | 23 | |
| % Within sex | 58.9 | 41.1 | |
| % Within IgG H. pylori | 78.6 | 62.2 | |
| Type of MS | 0.030 | ||
| Relapsing-remitting MS | |||
| Count | 20 | 10 | |
| % Within MS | 66.7 | 33.3 | |
| % Within IgG H. pylori | 76.9 | 83.3 | |
| Progressive | |||
| Count | 6 | 2 | |
| % Within MS | 75.0 | 25.0 | |
| % Within IgG H. pylori | 23.1 | 16.7 | |
5. Discussion
The present study aimed to assess the presence of H. pylori antibodies in patients with MS. Results showed significant differences between the MS patients and controls regarding the IgG antibodies. In 2003, Wender examined the association between H. pylori and MS. In this study, 90 patients with MS were studied serologically and for the presence of antibodies against H. pylori. The result was positive in 17 patients (18.9%), significantly lower than the prevalence of this infection in the normal population of Poland. The results showed no relation between MS and H. pylori (24). The results of our study contradict Wander's research. The most likely explanation for this discrepancy is the lack of a control group with specific demographic characteristics similar to the case group in Vander's study.
In 2012 a study was conducted by Ramroodi et al. to investigate the relationship between H. pylori and MS in southeastern Iran. In this case-control study, in 78 MS patients and 123 blood donors as a control group, bacterial DNA was detected in serum and saliva by RT PCR, and IgG antibodies against H. pylori (CagA and VacA antigens) were analyzed by Western blotting. Overall, the results did not show a relationship between MS and H. pylori infection (25). A study by Malli et al. in 2015 on 139 Indian patients showed a strong association between MS and the childhood infection of measles and no significant association between MS and the presence of H. pylori IgG antibodies (26).
In the next study by Gavalas et al. in Greece, researchers examined the prevalence of active H. pylori infection and abnormal endoscopic findings in MS patients. An endoscopy was performed on 44 patients with recurrent-healing MS and 20 patients with mild iron deficiency anemia to detect abnormal findings and active H. pylori infections confirmed by histological examination. H. pylori infection was reported in 86.4% of MS patients and 50% of anemic patients. This indicates that infection with H. pylori may contribute to MS progression (27). Gerges et al. reported a high level of anti-H. pylori heat shock proteins 60 (Hp hsp60) in MS patients (19). Using ELISA, Aboud et al. measured antibody levels of H. pylori IgG and IgA in 20 patients suffering from MS. They found a significant increase in the amount of H. pylori IgA compared with a control group (28).
In 550 patients with MS, Pedrini performed an enzyme immunoassay to detect specific IgG antibodies against H. pylori. These results reflect a protective effect of H. pylori on MS development in females (29). In the study published by Ranjbar et al. in Isfahan in 2019, the prevalence of H. pylori in MS patients and the level of pro-inflammatory and anti-inflammatory cytokines in the participants were measured. The 387 patients with relapsing-remitting MS as the case group and 420 healthy individuals as the control group were examined for IgG antibodies against H. pylori by ELISA. H. pylori infection rates were significantly lower in MS patients. On the other hand, in patients with MS and H. pylori, disability in terms of the EDSS scale was significantly less than in patients with MS who were seronegative. Also, in this group of patients, the level of pro-inflammatory cytokines such as IFN-γ, TNF-α, IL-6, and IL-17 was considerably lower, and the level of anti-inflammatory cytokines such as IL-4 and IL-10 was higher than seronegative individuals. Finally, they concluded that there was an inverse relationship between H. pylori infection and MS and that H. pylori could play a protective role (30). Ahadiat et al. reported MS patients had significantly lower levels of H. pylori IgG and IgA serum levels than controls and the protective effect of H. pylori infection in MS pathogenesis (31).
In the study published by Kountouras et al. in 2020, they hypothesized that one possible and compelling explanation for this connection is the phenomenon known as molecular mimicry. Helicobacter pylori can cause cross-reactivity with ganglioside surface components of peripheral and cerebral nerves, contributing to and potentially perpetuating the nerve tissue damage seen in diseases such as Guillain-Barré syndrome (GBS); damage to the surface membrane of Schwann cells or myelin leads to the development of GBS. Molecular mimicry of host structure by the saccharide fraction of lipopolysaccharides of the gastrointestinal pathogens Campylobacter jejuni and H. pylori appears to be involved in developing autoimmune sequelae (32).
On the other hand, in our study, the frequency of IgG antibodies against H. pylori in progressive MS was significantly higher than in the relapsing-remitting type. Despite some studies citing low antibody levels as the reason for the protective effect, more research concerning the details is needed.
5.1. Conclusions
Overall, in this study, the frequency of IgG antibodies against H. pylori was significantly higher in MS patients than in the control group. Also, this frequency in people with progressive MS was significantly higher than in recurrent-healing MS, which suggests the possible role of H. pylori in the development and progression of MS and can adversely affect the clinical course of the disease and its prognosis. Therefore, by considering H. pylori as a risk factor for MS, which has a high prevalence, and efforts to eradicate it in people at high risk for the disease (such as first-degree relatives of patients) and MS patients themselves, we may be able to reduce the incidence or progression of the disease and reduce the imposed costs to the community.
5.2. Limitations and Strengths Points and Weaknesses Points
In the current study, the sample size was limited due to time constraints. On the other hand, the lack of information on all patients for all variables was another limitation of the study. One of its strengths is that there is no difference between variables such as gender, age, and occupation in the case and control groups to have a minimal impact on the results. In addition, the effect of H. pylori infection on the clinical view of the disease was measured.