1. Background
Postoperative complications, including pain and sedation, have the potential to impede recovery, leading to delayed oral feeding and mobilization, thereby putting patients at risk for adverse outcomes such as delirium. The implementation of the enhanced recovery after surgery (ERAS) protocol, consisting of evidence-based interventions administered preoperatively, intraoperatively, and postoperatively (1), aims to mitigate these risks.
The implementation of the ERAS protocol aims to optimize physiological function, expedite postoperative recovery, alleviate pain intensity, and accelerate the initiation of oral feeding by minimizing patient stress before, during, and after surgery (2, 3). Utilizing the ERAS protocol has demonstrated a reduction in mortality rates, hastened recovery, shortened hospitalization durations, and improved overall surgical outcomes (4, 5). According to the ERAS protocol, appropriate management of preexisting comorbidities is crucial during the preoperative stage to prevent disease exacerbation following surgery. Additionally, healthcare providers play a vital role in guiding patients through self-management, symptom management, and postoperative complications (6). Educating preoperative patients regarding anticipated hospital stay duration, discharge criteria, and stress reduction techniques constitutes a fundamental aspect of this approach.
The prevalence of spine surgeries has shown a notable rise in the last decade (7). Following spine surgeries such as laminectomy, patients commonly experience significant pain in the initial postoperative days (8). Pain intensity and the administration of analgesics or sedatives can hinder patients' remobilization after surgery. Moreover, pain has been associated with an increased reliance on opioids for pain management (9, 10).
While intraoperative events did not exhibit a significant correlation with the length of hospital stay following spine surgeries, advanced age and comorbidities were found to be associated with longer stays (11). Originally designed to expedite hospital recovery, the ERAS protocol primarily focused on pain management, ileus prevention, patient resuscitation, and early discharge. However, it has been demonstrated that the ERAS protocol has the potential to accelerate remobilization time across various surgical procedures (12) and reduce the length of stay (LOS).
2. Objectives
The primary objective of this study is to investigate the impact of implementing an accelerated recovery method on remobilization initiation time compared to the control group following spinal surgery.
3. Methods
3.1. Study design
This study is a single-blind, randomized, controlled trial. Employing the 4-block randomization method, we randomly allocated 70 patients into two groups, with 35 patients in the control group and 35 patients in the ERAS group. The study received approval from the institutional review board (IRB) of the Tehran University of Medical Sciences (IR.TUMS.MEDICINE.REC.1400.129) and the Iranian Randomized Clinical Trial Committee (IRCT registration number: IRCT20211023052849N1).
3.2. Study Population and Sampling
Patients who provided consent and were eligible for elective spine surgery at Shariati Hospital, Tehran, were included in the study (Figure 1).
3.3. Inclusion and Exclusion Criteria
The study's inclusion criteria encompassed patients aged 18 to 80 years, who provided consent to participate, had ASA classes 1 to 3, underwent non-emergency spine surgery, and expressed willingness to participate.
Exclusion criteria for the study included emergency surgeries, lack of consent to participate, pregnancy, drug addiction, use of opioid drugs or their antagonists within 10 days prior to surgery, presence of liver or kidney diseases, history of psychiatric disorders, ischemic heart disease, cardiac dysrhythmia, hypertension, history of glaucoma or eye trauma, and porphyria.
3.4. Randomization and Blinding
To ensure random allocation of patients to treatment groups, the study utilized the 4-block method along with random numbers to generate a balanced and unbiased sequence of group assignments. Each patient was assigned to a block, representing either the control or intervention group, based on their assigned random number. The patients, monitoring-in-charge nurse, data collectors, and statistical analyzer were all blinded to the study's objectives.
3.5. Interventions and Monitoring
All patients underwent standard anesthesia monitoring following the ASA standards (13). The monitoring encompassed continuous electrocardiography (ECG), pulse rate assessment, oxygen saturation (SpO2) measurement, non-invasive blood pressure (NIBP) monitoring, and the measurement of carbon dioxide (CO2) levels in exhaled air after intubation (ETCO2).
The ERAS protocol, adopted from the recommendations of the ERAS Society (14), was implemented for the patients. Those in the ERAS group received interventions across three stages: preoperative, intraoperative, and postoperative care. Preoperative care focused on optimizing the patients' health status and lifestyle. The patients were provided with education regarding the surgery, hospitalization, and discharge process, as well as postoperative symptoms and restrictions. Any concerns or questions raised by patients were addressed accordingly. Smoking and alcohol consumption were discouraged starting one week before surgery. Patients with comorbidities (such as diabetes, hypertension, anemia, etc.) were referred to an internist for consultation. In the ERAS group, patients were permitted to consume carbohydrate-containing drinks up to two hours prior to the operation. On the other hand, patients in the control group were prepared for surgery using traditional methods and were instructed to observe a fasting period of at least eight hours before the surgery.
3.6. Anesthesia and Pain Management
Fluid therapy was conducted and sustained using crystalloids, colloids, and blood products (if necessary) to achieve and maintain a euvolemic state. The Pleth variability index (PVI) was monitored to assess fluid responsiveness, with a target range of 7 - 13, ensuring the adequacy of fluid therapy. The depth of anesthesia was assessed using a bispectral index (BIS) monitor. As a prophylactic measure against nausea and vomiting, all patients were administered intravenous (IV) Ondansetron at a dose of 4 mg.
All patients received propofol at a dose of 2 mg/kg, atracurium at a dose of 0.6 mg/kg, midazolam at a dose of 1 mg, and either fentanyl or sufentanil for the induction of general anesthesia. IV propofol infusion (50 - 150 mcg/kg/min) was utilized to maintain anesthesia in both groups, with the dosage adjusted based on the patient's depth of anesthesia and hemodynamic conditions. In cases of tachycardia and increased blood pressure, additional doses of fentanyl (50 - 100 mcg) or sufentanil (5 - 10 mcg) were administered during the surgery.
A Visual Analog Scale (VAS) was employed to evaluate pain intensity following surgery (15, 16). This patient-reported scale assigns a value of 0 to indicate no pain and a value of 10 to represent the most severe pain. For postoperative pain management, IV acetaminophen at a dose of 1 g was administered every eight hours for a duration of 48 hours. In cases where the VAS score exceeded 4, fentanyl was prescribed at a dose of 50 - 100 micrograms until the pain level on the VAS scale dropped below 4.
Preemptive analgesia was implemented with oral gabapentin administered on the night before the surgery at a dose of 200 mg. The postoperative pain management strategy for the ERAS group involved the administration of IV acetaminophen (1 g) in the post-anesthesia care unit (PACU), followed by oral acetaminophen (1 g) every 6 hours. Fentanyl (50 mcg) was utilized until the pain level on the VAS scale decreased to below 4. Intraoperatively, the ERAS group received two infusions to maintain analgesia. This included a ketamine IV bolus of 0.5 mg/kg followed by a continuous infusion of 0.25 mg/kg/h, administered up to 3 minutes before the conclusion of surgery. Additionally, an IV bolus of lidocaine at a dose of 100 mg, followed by a continuous infusion of 1 mg/kg/h, was administered. The liberal use of opioids was not permitted in this group.
The control group followed the standard hospital protocol, which included maintaining a "nothing by mouth" (NPO) status for 8 hours before surgery, with a light dinner. As per the hospital's routine protocol, oral administration of Librium (10 mg) was given the night before surgery for sedation purposes. In terms of intraoperative pain management in the control group (C), IV fentanyl (50 - 100 mcg) was administered at intervals of 20 - 30 minutes. Additionally, if tachycardia or an increase in blood pressure by more than 25% of the pre-anesthesia induction value occurred, a bolus dose of IV fentanyl (50 mcg) was administered. Foley catheter insertion was performed as a routine procedure in the control group. Postoperative pain management in the control group involved the use of patient-controlled analgesia (PCA) with fentanyl.
3.7. Statistical Analysis
SPSS version 26 was utilized for data analysis. Descriptive statistics were used to report quantitative variables, presenting the mean and standard deviation, while qualitative variables were reported in terms of frequency and percentage. Changes in the patients' pain intensity over time were examined using the repeated-measures ANOVA test. The independent t-test was employed to compare numerical variables between the ERAS and control groups. The chi-square test (or Fisher's exact test) was applied to assess the relationship between nominal (or categorical) variables. Multiple regression analysis was conducted to identify predictors of remobilization time. A significance level of less than 5% (P < 0.05) was considered statistically significant.
4. Results
A total of 70 patients were enrolled in this study, with 52.9% being women and a mean age of 47.56 ± 14.08 years (Table 1).
| Variables | Group (n = 70) | ||
|---|---|---|---|
| Control (n = 35) | ERAS (n = 35) | P Value | |
| Gender | 0.811 | ||
| Male | 16 (48.5) | 17 (51.5) | |
| Female | 19 (51.4) | 18 (48.6) | |
| Surgery location | 0.865 | ||
| Thoracic laminectomy | 3 (42.9) | 4 (57.1) | |
| Lumbar laminectomy | 26 (52.0) | 24 (48.0) | |
| Cervical laminectomy | 6 (46.2) | 7 (53.8) | |
| Postoperative nausea and vomiting (PONV) | 0.047 | ||
| No | 26 (44.8) | 32 (55.2) | |
| Yes | 9 (75.0) | 3 (25.0) | |
| Use of Opioids for postoperative analgesia | < 0.001 | ||
| No | 0 (0.0) | 17 (100.0) | |
| Yes | 35 (66.0) | 18 (34.0) | |
| Other postoperative complications | 0.999 | ||
| No | 35 (50.0) | 35 (50.0) | |
| Yes | 0 (0.0) | 0 (0.0) | |
| Initiation oral feeding within 6 hours after surgery | 0.001 | ||
| No | 20 (76.9) | 6 (23.1) | |
| Yes | 15 (34.1) | 29 (65.9) | |
| Pain intensity after surgery (recovery time) | < 0.001 | ||
| VAS ≤ 4 | 7 (21.9) | 25 (78.1) | |
| VAS > 4 | 28 (73.7) | 10 (26.3) | |
| Pain intensity 1 hour after surgery | < 0.001 | ||
| VAS ≤ 4 | 13 (27.7) | 34 (72.3) | |
| VAS > 4 | 22 (95.7) | 1 (4.3) | |
| Pain intensity 6 hours after surgery | 0.999 | ||
| VAS ≤ 4 | 35 (50.0) | 35 (50.0) | |
| VAS > 4 | 0 (0.0) | 0 (0.0) | |
| Age | 48.14 ± 13.89 | 46.97 ± 14.43 | 0.340 |
| Body mass index (BMI) | 21.94 ± 3.70 | 21.97 ± 3.29 | 0.970 |
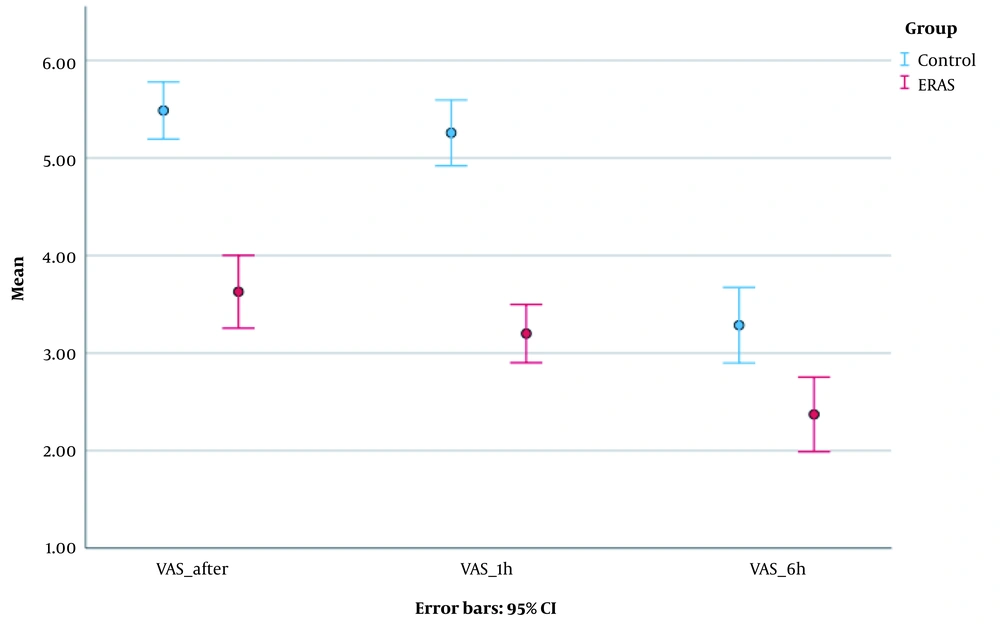
| VAS after surgery (recovery time) | 5.49 ± 0.85 | 3.63 ± 1.09 | < 0.001 |
| VAS after 1 hour | 5.26 ± .98 | 3.20 ± .89 | < 0.001 |
| VAS after 6 hours | 3.29 ± 1.13 | 2.37 ± 1.11 | 0.001 |
| Opioid Fentanyl dose (mcg) | 255.00± 40.74 | 108.33 ± 49.26 | 0.004 |
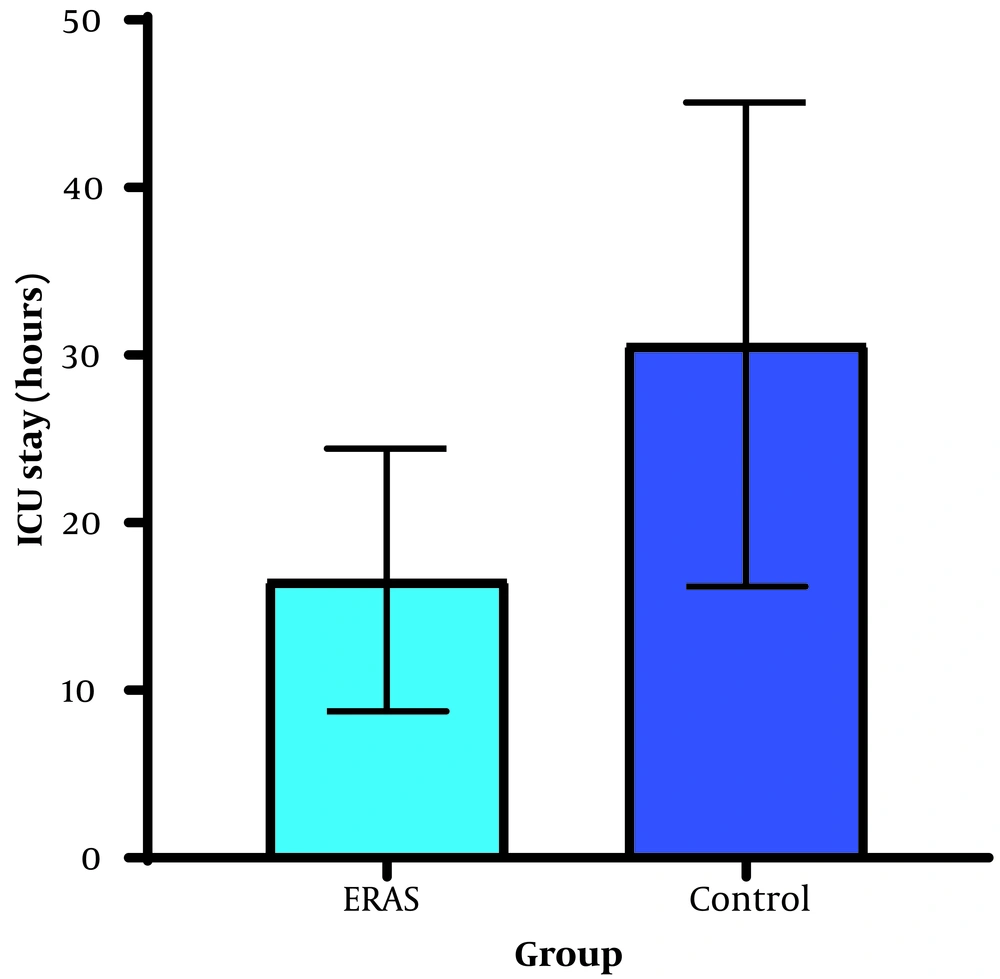
| ICU stay (hours) | 26.74 ± 7.18 | 14.06 ± 3.01 | < 0.001 |
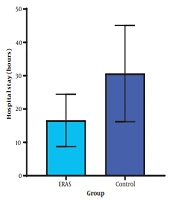
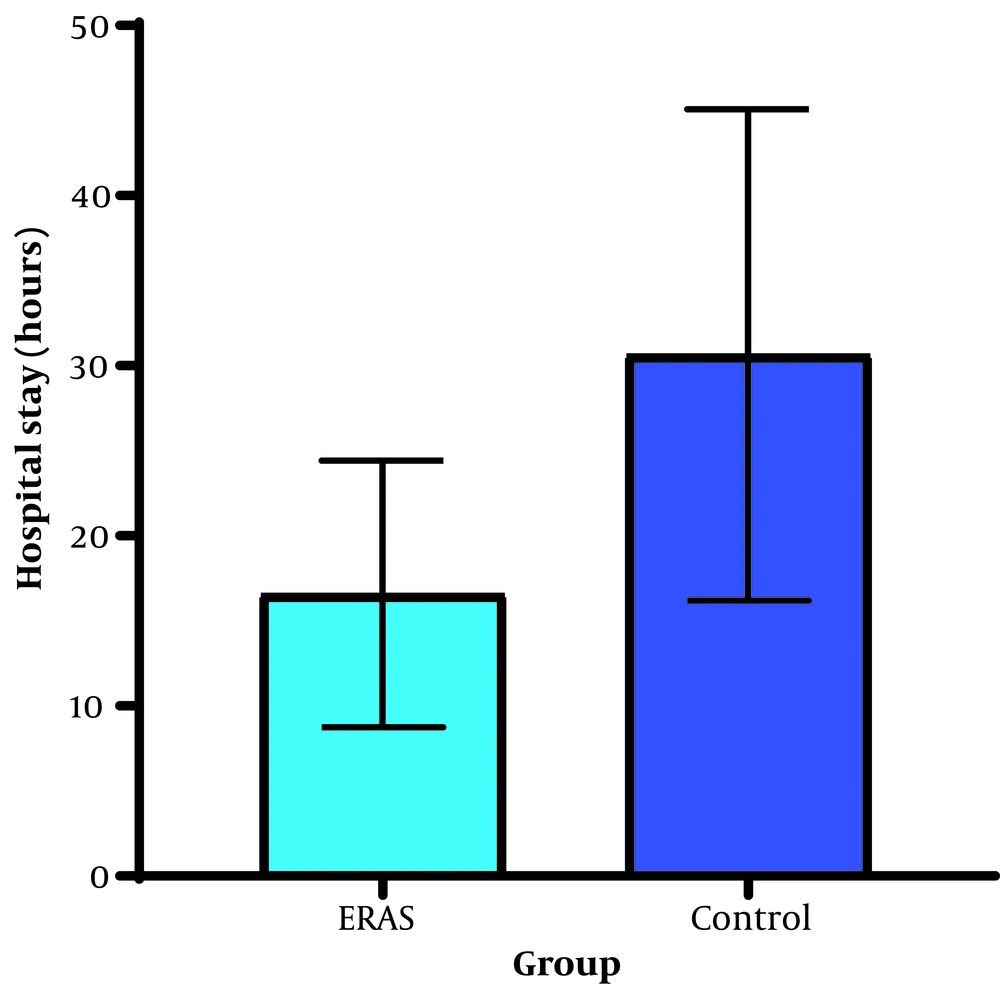
| Hospital stays (hours) | 46.63 ± 6.99 | 25.37 ± 3.87 | < 0.001 |
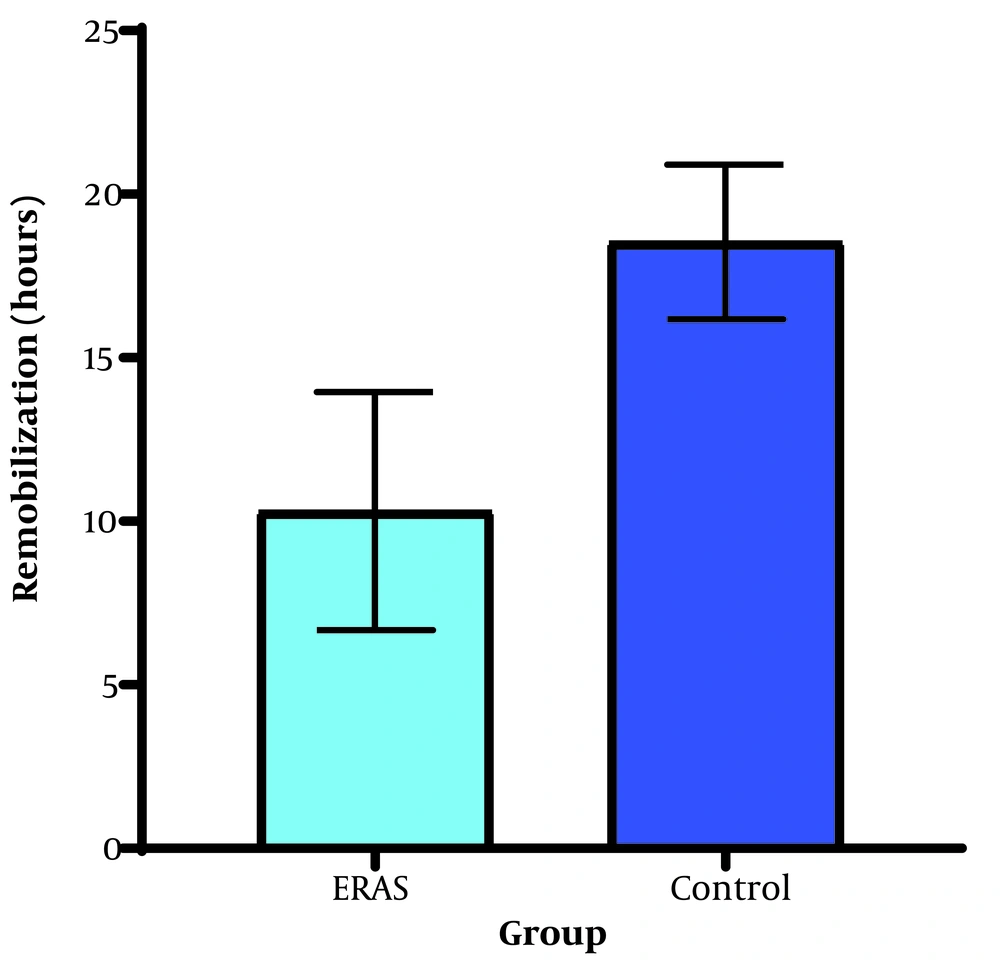
| Remobilization after surgery (hours) | 18.54 ± 2.36 | 10.31 ± 3.64 | < 0.001 |
| Systolic blood pressure (SBP) after surgery (mmHg) | 95.69 ± 9.15 | 101.31 ± 17.22 | 0.097 |
a Values are expressed as No. (%) or mean ± SD.
Age, BMI, and gender distribution did not differ significantly between the two groups. The control group exhibited a significantly higher occurrence of postoperative nausea and vomiting compared to the ERAS group (n = 9 vs. 3, P = 0.047). The use of narcotics for postoperative pain management was more prevalent in the control group (P < 0.001). No other postoperative complications were observed. A greater proportion of patients in the ERAS group (n = 29, 65.9%) initiated oral nutrition within six hours after surgery, which was significantly higher than the control group (n = 15, 34.1%, P = 0.001). While the trend of pain intensity (VAS) decreased in all patients after surgery, a majority of patients in the control group reported higher levels of pain (VAS > 4) immediately after surgery (n = 28, 73.7%, P < 0.001) and one hour later (n = 22, 95.7%, P < 0.001), indicating statistical significance. However, six hours after surgery, no patients reported a VAS score higher than 4 (P = 1).
The mean reported pain levels immediately after surgery (3.63 vs. 5.49, P < 0.001), one hour after surgery (3.20 vs. 5.26, P < 0.001), and six hours after surgery (2.37 vs. 3.29, P = 0.001) were significantly lower in the ERAS group. The dose of opioids (Fentanyl) used for postoperative analgesia was significantly lower in the ERAS group (P = 0.004) (Figure 2). The ERAS group had significantly shorter durations of ICU stay (14.06 vs. 26.74, P < 0.001), hospital stay (25.37 vs. 46.63, P < 0.001), and remobilization time after surgery (10.31 vs. 18.54, P < 0.001) compared to the control group (Figures 3 and 4).
A repeated measures ANOVA test was utilized to assess pain intensity at the measured intervals following surgery (Figure 5).
The pain intensity differed significantly at the three measured times (P < 0.001). The overall trend of pain reduction was observed in both groups, with the ERAS group showing a significantly faster rate of improvement. The statistical model yielded a medium effect size (η2 = 0.106, λ = 0.171), indicating that the ERAS protocol has a moderate to large effect in reducing postoperative pain. This is further supported by the significant impact of the ERAS protocol on pain reduction (Figure 5). Remobilization time correlated significantly with pain intensity immediately after surgery (r = 0.651, P < 0.001), pain intensity one hour after surgery (r = 0.723, P < 0.001), pain intensity six hours after surgery (r = 0.391, P = 0.001), fentanyl dose (r = 0.728, P < 0.001), and length of hospital stay (r = 0.727, P < 0.001). Multiple regression analysis revealed that pain intensity one hour after surgery, fentanyl dose, and hospital stay significantly predicted remobilization time (F (1,68) = 8.036, P < 0.001) (Table 2).
| Variables | Unstandardized β | t | P Value |
|---|---|---|---|
| Overall model | 8.239 [0.744 – 15.733] | 2.199 | 0.032 |
| Pain intensity (VAS) after 1 h | 0.872 [0.158 – 1.585] | 2.444 | 0.017 |
| Opioid Fentanyl dose | 0.038 [0.023 – 0.053] | 5.048 | < 0.001 |
| Hospital stays (hours) | 0.164 [0.088 – 0.240] | 4.303 | < 0.001 |
| Age | -0.023 [-0.071 – 0.024] | -0.977 | 0.332 |
| BMI | -0.085 [-0.274 – 0.104] | -0.900 | 0.372 |
| PONV | 0.304 [-1.556 – 2.163] | 0.327 | 0.745 |
| VAS after surgery | 0.022 [-0.699 – 0.743] | 0.061 | 0.952 |
| VAS after 6h | -0.468 [-1.118 – 0.022] | -1.441 | 0.155 |
| SBP after | -0.026 [-0.075 – 0.022] | -1.082 | 0.284 |
5. Discussion
The ERAS protocol is a comprehensive care pathway that encompasses preoperative, intraoperative, and postoperative phases, with the goal of promoting early recovery and minimizing the physiological stress response following surgery (1). Essential components of the ERAS protocol include preoperative education, optimizing nutrition, standardized pain management and anesthesia, and early mobilization (17). This study aimed to assess the impact of implementing an accelerated recovery method on time to restart mobilization in patients undergoing spinal surgery, compared to a control group.
The primary finding of this study highlights a significant reduction in remobilization time (in hours) among patients in the ERAS group compared to the control group. Both groups experienced a decrease in postoperative pain within 6 hours after surgery, but the reduction was significantly more pronounced in the ERAS group.
Previous studies have demonstrated the potential of the ERAS protocol to reduce hospital stay duration (18). Consistent with these findings, our study showed that patients receiving ERAS protocol care experienced significantly lower opioid use and postoperative pain intensity without any significant increase in adverse events or readmissions. Complication rates associated with the ERAS protocol have been reported to range from 2.0% to 31.7%. In our study, the implementation of the ERAS protocol for spine surgery candidates led to a reduction in both the intensive care unit and overall hospital stay. Additionally, the ERAS group demonstrated significantly reduced pain compared to the control group. The use of ERAS in spine surgery holds promise for minimizing complications, readmissions, length of stay, and opioid consumption while enhancing patient-reported outcomes and functional recovery.
Wainwright et al. (19) proposed that in light of increasing surgical costs and patient dissatisfaction, implementing an ERAS protocol that prioritizes evidence-based practices and streamlined logistics can facilitate faster recovery and reduce complications for spine surgery patients. Furthermore, adopting this protocol has the potential to enhance long-term outcomes.
The findings of a systematic review (20) confirm that the ERAS protocol yields significant benefits, including shorter hospital stays, decreased complications, and reduced postoperative pain. These advantages are consistent across various surgical categories, such as spine surgeries, orthopedic surgeries, and cosmetic surgeries. Our study's results align with these findings, with lumbar surgery being the most common procedure in both patient groups. Although cervical spine surgeries represented a small proportion of our study population, the overall concept of ERAS, focused on minimizing complications and pain and subsequently reducing hospital stays, appears to be applicable to spine surgery.
The findings of the study conducted by Soffin et al. (21) demonstrated a protocol compliance rate of 85.03 percent. The average duration of hospital stay was 279 minutes, and no association was found between the type or duration of surgery and the length of hospitalization. Similarly, our study did not identify any such correlation. Approximately 37% of the participants had a preexisting tolerance to opioids at the time of surgery. However, no significant impact of initial opioid use on the length of hospital stay or the total amount of intraoperative or post-anesthesia care unit (PACU) narcotic usage was observed. Furthermore, it has been recognized that the implementation of the ERAS protocol is crucial in spine surgery, as patient recovery in this context is often protracted, painful, costly, and characterized by substantial variability (22).
The hospital stay duration in our study was 46 hours for the control group and 24 hours for the ERAS group. A study conducted by d'Astorg et al. (23) also reported a significant disparity in the average hospitalization duration, referring to the average number of days spent in the hospital, between the two groups. However, despite these variations, both groups exhibited similar rates of complications, re-hospitalization, postoperative pain, performance, and satisfaction. These findings deviated slightly from the results of our study, possibly due to differences in the statistical population size between our study and the study by d'Astorg et al. (23).
The primary finding of our study focused on comparing the restart duration of patients between the two groups. Our results demonstrate that implementing ERAS not only offers the mentioned benefits but also decreases the time it takes for patients to restart. This aspect holds significant importance in spine surgeries as it helps mitigate the potential negative effects of prolonged bed rest post-surgery, such as deep vein thrombosis or pulmonary embolism. Since no similar study has been conducted in this particular area, this finding should be considered preliminary.
In the final analysis, it was determined that remobilization time could be predicted based on hospital stay (in hours), pain intensity one hour after surgery, and the dosage of Fentanyl administered for postoperative pain management. A longer hospital stay correlated with an extended remobilization time. The clinical interpretation of this finding presents some challenges since patients undergoing spine surgery are first mobilized and then discharged from the hospital. Consequently, patients requiring more time to remobilize will be discharged later. Additionally, an increase in prescribed Fentanyl post-surgery and higher pain intensity one hour after the procedure were associated with a longer remobilization time. Essentially, patients who received higher doses of Fentanyl for pain relief but still experienced more intense pain one hour after surgery required an extended remobilization time. This discovery underscores the significance of effective pain control in patients undergoing spine surgery, as it significantly impacts the time it takes for patients to resume mobility. This finding aligns with a recent meta-analysis, which highlighted that regardless of opiate use, lower pain ratings are linked to improved mobility.
5.1. Limitation
This study had two primary limitations. Firstly, it was conducted at a single center, which makes it challenging to generalize the findings to a broader population. Secondly, the sample size of the treated group was small, necessitating a larger sample size to validate the results. Lastly, the intervention patients included in this study were limited to non-emergency surgery cases.
5.2. Conclusion
The application of the ERAS protocol in spine surgery may reduce the length of stay and opioid consumption, along with improving the time to remobilize patients. The level of pain one hour after surgery, the Fentanyl dose (given as an analgesic), and the length of hospital stay can all predict remobilization time after elective spinal surgery.