1. Background
Cerebral venous sinus thrombosis (CVST) is a rare cause of stroke and a potentially life-threatening condition with a mortality rate of 1.327 per 100,000 population (1, 2). Its prevalence is three times higher in women than men (3). Diverse and non-specific symptoms with a wide range of clinical presentations make the diagnosis challenging and may even result in missed diagnosis due to the indistinct nature of the disease (2, 3). The most common presentation is mainly localized headache (89%). Moreover, about 10% of patients suffer from focal neurological deficits during the first year after the onset of stroke, which reduces their quality of life as morbidity (1). Coma (a Glasgow Coma Scale score < 9), seizure, and mental status changes are important predictors of death in the first 30 days after the disease onset (4).
The main risk factors of CVST include thrombocytosis (polycythemia), hemodynamic changes (stasis or turbulence of blood flow), vascular endothelial damage, pregnancy, oral contraceptive pills consumption, anticoagulation therapy, lumbar puncture, blood disorders, like myeloproliferative diseases, hereditary thrombophilia, hyperhomocysteinemia, and paroxysmal nocturnal hemoglobinuria (PNH) (5). Different studies have demonstrated an association between intravenous sinus thrombosis and iron deficiency anemia (IDA), and several mechanisms have been proposed to explain this relationship (6, 7). Iron is considered an important regulator of thrombopoiesis, and its deficiency can lead to thrombocytosis. Iron deficiency can cause a hypercoagulable state by altering the blood flow pattern in the vessels. It has been suggested that areas prone to hypoxic injury in the brain (basal ganglia and thalamus) may be affected by poor oxygenation due to IDA (8, 9). On the other hand, ferritin is the most important protein that stores iron in the body, and its low levels can be an indicator of IDA.
Nevertheless, conditions such as inflammation, malignancy, obesity, and infection can increase ferritin levels, which may mask underlying iron deficiency (10, 11). In other words, ferritin, along with erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP), is an acute-phase reactant with increased levels in numerous inflammatory conditions and may be very helpful in making a diagnosis or evaluating prognosis in different clinical and para-clinical settings (10). As a result, due to the inflammatory nature of CVST, ferritin levels may increase as an acute-phase reactant.
Previous studies have demonstrated a significant direct correlation between serum levels of ferritin and acute myocardial infarction (12). Ferritin can exacerbate the low-density lipoprotein (LDL) oxidation process, atherosclerosis, and thrombosis formation resulting in further cardiovascular complications (13).
2. Objectives
The current study aims to investigate the serum levels of ferritin, iron, and TIBC in CVST patients and their possible relationship with different parameters, such as oral contraceptive pills consumption, the history of CVST or CVA, underlying diseases, and involved cerebral venous sinus.
3. Methods
3.1. Study Design and Subjects
Participants of this descriptive study were 30 patients with documented CVST, and the study duration was one year. Patients were selected from individuals with CVST symptoms who visited Vali-e-Asr Zanjan University Hospital within the first 24 hours of symptom onset.
The study was approved by Zanjan University Ethical Committee (code: A-12-205-17), and informed consent was collected from patients or their legal representatives. The criteria of ethics for conducting this study were as follows:
(1) All participants must take part voluntarily and free from any influences, and their rights and autonomy should be respected and adequately protected;
(2) Research staff and participants should be given appropriate information about the study comprehensibly. The information should include the research procedure, the purposes, risks, and anticipated benefits, and a statement offering the participant the opportunity to ask questions and withdraw at any time from the research;
(3) Research should be worthwhile and provide value that outweighs any risk or harm;
(4) Data generated by research must be securely stored following relevant legislation and institutional policy;
(5) The independence of research should be clear, and any conflicts of interest or partiality should be explicit.
All patients were examined carefully, and the diagnosis was supported by the presence of typical CVST symptoms along with brain magnetic resonance imaging (MRI) and magnetic resonance venography (MRV) findings. The most important finding for diagnosis confirmation was the lack of intravenous blood flow with the presence of an intraluminal thrombosis on MRI with gadolinium and MRV and negative neuroimaging for any other focal neurological deficits.
Patients with concomitant or history of liver diseases, malignancies, meningoencephalitis, focal neuroimaging, and physical examination findings leading to another diagnosis and those who did not consent to participate in the study were excluded.
3.2. Data Collection
A blood sample (5 cc) was drawn from each patient by a trained emergency department (ED) nurse within the first 24 hours of symptom onset and a definitive diagnosis of CVST. Blood samples were delivered to the hospital laboratory, and extracted plasma samples were stored at -70°C in the Vali-e-Asr hospital laboratory. After data collection, a total of 30 plasma samples were analyzed for ferritin levels using electrochemiluminescence immunoassays (ECLIA) (Roche Diagnostics GmbH, Mannheim, Germany) and iron and TIBC levels using the calorimetric method (Biorexfars, Fars, Iran) according to the manufacturer’s instructions.
Ferritin values of the reference laboratory range from 30 - 400 ng/mL for men and 15 - 180 ng/mL for women. Serum iron values of the reference laboratory range from 65 - 176 μg/dL in men and 50 - 170 μg/dL in women, and TIBC values of the reference laboratory range from 240 - 450 μ/dL for both men and women.
3.3. Statistical Analysis
Descriptive statistics were reported as mean ± standard deviation (SD) and number (percent) as applicable. The normality of the data was examined using the Kolmogorov-Simonov test. A P-value less than 0.05 was regarded as statistically significant, and IBM SPSS 18.0 software for Windows was used for statistical analysis.
The mean serum ferritin, iron, and TIBC levels were compared between dichotomous variables using independent samples t-test. Analysis of variance was used to compare the mean of serum ferritin, iron, and TIBC levels between three or more groups.
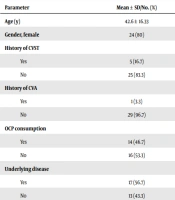
After complete evaluations, a list consisting of the patient’s age, gender, underlying diseases history (diabetes, hypertension, cardiovascular disease, CVST, etc.), oral contraceptive pill (OCP) consumption in the past four months, pregnancy state, involved cerebral venous sinus (transverse, superior sagittal, and sigmoid sinus) and mean serum levels of ferritin, iron, and TIBC, was assembled for final inclusion in data set (Table 1).
| Parameter | Values a |
|---|---|
| Age (y) | 42.6 ± 16.33 |
| Gender, female | 24 (80) |
| History of CVST | |
| Yes | 5 (16.7) |
| No | 25 (83.3) |
| History of CVA | |
| Yes | 1 (3.3) |
| No | 29 (96.7) |
| OCP consumption | |
| Yes | 14 (46.7) |
| No | 16 (53.3) |
| Underlying disease | |
| Yes | 17 (56.7) |
| No | 13 (43.3) |
| Involved sinus | |
| Transverse | 26 (86.7) |
| Superior sagittal | 2 (6.7) |
| Sigmoid | 2 (6.7) |
| Pregnancy, positive | 2 (6.7) |
| Serum ferritin (ng/mL) | 87.36 ± 88.84 |
| Normal | 24 (80) |
| Low | 6 (20) |
| Serum iron (mg/dL) | 99.40 ± 29.97 |
| Normal | 27 (90) |
| Low | 3 (10) |
| TIBC (mg/dL) | 324.5 ± 49.57 |
| Normal | 29 (96.7) |
| Low | 1 (3.3) |
Abbreviations: CVST, cerebral venous sinus thrombosis; CVA, cerebrovascular accident; OCP, oral contraceptive pill; TIBC, total iron binding capacity
a Values are expressed as mean ± SD or No. (%).
4. Results
A total of 30 patients (six men and 24 women) with a definitive diagnosis of CVST were included in the analysis, and the mean age of participants at the time of sampling was 42.6 ± 16.33 years. The mean serum levels of ferritin, iron, and TIBC were 87.36 ± 88.84 ng/mL, 99.40 ± 29.97 mg/dL, and 324.50 ± 49.57 mg/dL, respectively (Table 1).
The mean serum iron levels were 93.54 ± 29.7 mg/dL in women and 122.83 ± 18.18 mg/dL in men, and the mean serum TIBC levels were 332.25 ± 52.40 mg/dL in women and 293.5 ± 13.47 mg/dL in men group. Although the mean serum levels of iron and TIBC were lower and higher among women than men, the results were in reference laboratory ranges. Therefore, we attribute this difference to lower serum iron levels in women due to menstrual cycles.
We then measured mean serum levels of ferritin, iron, and TIBC according to the different variables. Results showed no significant relationship between ferritin, iron, and TIBC serum levels and OCP consumption in the past four months, involving sinus with thrombosis and positive history of CVA, CVST, or other underlying diseases (P-value < 0.05) (Table 2).
| Variable | Ferritin | Iron | TIBC |
|---|---|---|---|
| Gender | |||
| Female | 68.03 ± 53.68 | 93.54 ± 29.7 | 332.25 ± 52.40 |
| Male | 164.71 ± 153.47 | 122.83 ± 18.18 | 293.5 ± 13.47 |
| P-value b | 0.186 | 0.03 | 0.003 |
| History of CVST | |||
| Yes | 68.88 ± 61.74 | 96.56 ± 31.33 | 330.36 ± 73.51 |
| No | 91.06 ± 93.9 | 113.6 ± 17.98 | 295.2 ± 21.71 |
| P-value b | 0.61 | 0.25 | 0.15 |
| History of CVA | |||
| Yes | 95 | 120 | 297 |
| No | 87.10 ± 90.4 | 97.68 ± 30.25 | 325.44 ± 50.17 |
| P-value b | 0.93 | 0.49 | 0.58 |
| OCP consumption | |||
| Yes | 58.42 ± 44.29 | 89 ± 32.71 | 343.35 ± 58.34 |
| No | 112.69 ± 109.97 | 108.5 ± 24.91 | 308 ± 34.33 |
| P-value b | 0.09 | 0.07 | 0.06 |
| Underlying diseases | |||
| Yes | 77.17 ± 58.33 | 100.29 ± 29.80 | 322.88 ± 51.03 |
| No | 100.7 ± 119.15 | 98.23 ± 31.38 | 326.61 ± 49.57 |
| P-value b | 0.48 | 0.85 | 0.84 |
| Involved sinus | |||
| Transverse | 91.45 ± 93.30 | 99.26 ± 28.91 | 322.46 ± 44.66 |
| Superior sagittal | 104 ± 12.72 | 114 ± 8.48 | 306 ± 12.72 |
| Sigmoid | 17.6 ± 19.79 | 86.5 ± 99.26 | 369.5 ± 127.98 |
| P-value b | 0.52 | 0.67 | 0.98 |
Abbreviations: CVT, cerebral venous thrombosis; CVA, cerebrovascular accident; OCP, oral contraceptive pill; TIBC, total iron binding capacity
a Values are expressed as mean ± SD.
b P-value < 0.05
To determine if the mean values of serum ferritin, iron, and TIBC are significantly different from the normal population’s mean levels, we compared the results of our study with the results of a similar regional study from healthy individuals using one-sample t-test (Table 3) (14).
| Variable | Present Study | Population-based Study | t |
|---|---|---|---|
| Serum Ferritin | 87.36 ± 88.84 | 69.5 ± 54.7 | -0.3 |
| Serum iron | 99.4 ± 29.97 | 102.4 ± 44.7 | -0.54 |
| TIBC | 324.5 ± 49.57 | 437.8 ± 67.5 | -13.56 b |
Abbreviation: TIBC, total iron binding capacity
a Values are expressed as mean ± SD.
b P-value < 0.01
5. Discussion
Results revealed normal serum levels of ferritin, iron, and TIBC among the study participants. Additionally, we evaluated the possible relationship between serum levels of ferritin, iron, and TIBC with different parameters, and there were no statically significant associations with OCP consumption within the past four months, involved cerebral venous sinus, and history of CVA, CVT or other underlying diseases.
Limited studies have evaluated ferritin and other iron serum indices levels in CVST for possible prognostic or preventative benefits with controversial results.
Silvis et al. studied mean hemoglobin (Hb) levels in 952 patients with CVST, 22% of whom had anemia on admission (15). Results showed that the risk of poor clinical outcomes in patients with anemia on admission nearly doubled in patients with mild and patients with moderate to severe anemia. It is thought that anemia induces hyperdynamic circulation, which triggers an inflammatory response and increases thrombus formation (15).
Liu et al. assessed 238 patients with cerebral venous thrombosis, among whom 73 were diagnosed with anemia, and concluded that severe and microcytic anemia was independently associated with an increased risk of CVST and higher mortality rates (6). Therefore, anemia may be an independent predictor of unfavorable functional outcomes in CVST patients (6). Various studies have also suggested this association (16-18).
Although these findings do not support the results of our study, we believe that inflammation in CVST may result in increased ferritin levels as an acute phase reactant and mask the underlying iron deficiency in early evaluations (10, 11). This can be adjusted by early utilization of other diagnostic indices for IDA (such as hemoglobin levels).
Northrop, in a review study, suggested that although naturally synthesized ferritin and transferrin receptor proteins are regulated by intracellular iron concentrations, increased serum ferritin level during inflammation is similar to the positive acute-phase reactant, CRP, and is probably induced by inflammatory cytokines (independent from intracellular iron concentrations) (19). The mean serum levels of ferritin among the current study population were higher than their levels in a similar regional study of healthy individuals, which is congruent with Northrop-Clewes’s review findings (19).
Positive associations between serum ferritin levels and cardiovascular diseases have been demonstrated and can be explained through different mechanisms (12). Duarte et al., in a cohort study (during two years) of 280 patients with acute coronary syndrome (ACS) diagnosis, suggested that adverse cardiovascular events are associated with higher serum ferritin levels, which also can be an independent predictor of long-term mortality (20). Another mechanism for this association is that increases in serum ferritin levels enhance the oxidation of LDL-cholesterol, which further induces blood vessel inflammation and atherosclerosis progression due to the pro-oxidant properties of ferritin (21). According to the “iron hypothesis” by You and Wang, iron depletion can reduce the risk of myocardial infarction (MI) and ACS, and high iron stores were considered atherogenic (13). Although this relation is still controversial and requires further investigation, it is noteworthy that the association between atherosclerotic lesions and venous thrombosis has been well established (13).
Similarities between atherosclerosis and thrombosis formation mechanisms in cardiovascular diseases with venous sinus thrombosis formation in CVST as an inflammatory condition support our hypothesis on the possible association between serum ferritin and other iron indices with CVST; however, no statistically significant relationship was found in our study.
5.1. Conclusions
Among patients presenting with CVST, serum ferritin, iron, and TIBC mean levels were normal. Although ferritin levels were higher among patients than their mean levels in a similar regional study of healthy individuals, this difference was not statically significant to show the possible benefits of utilizing these indices. Furthermore, there was no statically significant association between iron indices and study parameters (recent OCP consumption, involved sinus, history of CVA, CVT, and other underlying diseases).
5.2. Limitations
Although our sample size was small (30 patients) due to the rare nature of this disease, current evidence may present preliminary data on the controversial levels of serum ferritin and other iron indices in CVST. Further studies are needed to increase our knowledge.
We suggest case-control studies with more participants or multicentric studies with larger sample sizes. Studies that evaluate this relationship, including other blood indices, such as hemoglobin (even during the follow-up period), are also recommended.