1. Background
Aging is the irreversible change in the physiological processes at the cellular level. Aging is associated with neurodegenerative diseases, cardiovascular disease, cancer, and diabetes (1, 2). Various mechanisms have been proposed to explain aging, such as those related to reactive oxygen species (ROS), mTOR involvement, brain structural changes, inflammation, apoptosis, and neuroendocrine dysfunction (3, 4).
Oxidative stress and inflammation are involved in spatial memory loss and brain hippocampal tissue damage (5). Anxiety-like behavior has been associated with an increase in ROS (6). Brain-derived neurotrophic factor (BDNF), a neurodevelopmental factor, plays a regulatory role in synaptic formation, neuronal differentiation, and various psychiatric disorders pathogenesis (7). The hippocampus and cerebral cortex possess a high level of BDNF (8). Low levels of BDNF accompanied by aging may contribute to memory decline (9).
Severe and persistent stress could be a harmful condition for body function (10). Chronic variable stress (CVS) changes the secretion of neurotransmitters and structural changes in the neural network, such as the hippocampus, prefrontal cortex, and amygdala (11). Changes in prefrontal cortex cells can cause mental disorders such as depression (12). Furthermore, cognitive disorders caused by chronic stress can occur due to molecular and morphological changes in the hippocampus and parahippocampal areas of the brain (13). Adrenal hypertrophy and high levels of cortisol and other glucocorticosteroids occur during chronic stress (14). These higher levels of glucocorticoids are life-saving in short periods. However, cortisol and other glucocorticoids have harmful and life-threatening effects on humans and perhaps mammals if their blood levels remain high during chronic stress. For example, they accelerate aging, increase the chance of diabetes and cardiovascular diseases, and reduce the activity of the immune system (15).
Metformin, a drug for type 2 diabetes mellitus, has been studied for other pathologic conditions, such as neurodegenerative diseases, including Parkinson's disease and Alzheimer's disease, which are a major concern in the aging population (16). Data revealed that metformin could prevent aging-related diseases, including cardiovascular disease, cancer, and neurobehavioral dysfunctions, by antioxidant mechanisms (17). Metformin could play an antiaging role in the brains of accelerated senescence and aged animal models (18). Metformin has been shown to oppose stress-induced depression by enhancing BDNF expression (19). Data revealed that metformin could alleviate the neurocognitive deficit in animal aging models through neuroinflammation improvement, apoptosis reduction, synaptic plasticity promotion, and oxidative stress attenuation via activation of the AMPK/BDNF/PI3K signaling pathway (20). Moreover, metformin diminished the stress-induced cellular senescence of aging human adipose stromal cells and could improve age-related adipocyte dysfunction (21). As there are not enough experiments on the role of metformin in stressful aging, investigating the effects of metformin on aging parameters under chronic stress could be of great value.
2. Objectives
According to previous studies, we selected the chronic stress model in aging rats and assessed the impact of metformin under these circumstances. We attempted to analyze this medication from a comprehensive standpoint, encompassing not only neurological disorders but also muscular disorders and potential mechanisms of action.
3. Methods
3.1. Materials
Metformin was obtained from Merck Pharmaceuticals (Merck, France). Metformin was administered orally (gavage) at the doses of 1 and 10 mg/kg (20). The normal saline was used as a metformin vehicle. Brain BDNF protein levels were measured by the ELISA method using a commercial kit (Zellbio, Germany). Superoxide dismutase (SOD) enzyme activity assay kits (ZellBio, Germany) were applied to determine the antioxidant levels in brain tissue.
3.2. Animals
This study used aged male Wistar rats (300 - 350 g, 18 months, N = 48). The animals were randomly divided into six groups (N = 8) by a person blind to the study and kept under the standard dark/light cycle (lights on from 7:00 to 19:00), temperature (22 ± 2°C), and free access to water and food. This research was done under the local ethics code (IR.RUMS.REC.1397.050) and according to standard ethical guidelines (NIH, publication no. 85-23, revised 1985; European Communities Directive 86/609/EEC). The aged animals were randomly divided into six groups as follows: Group 1: Control (normal aged animals); groups 2 and 3: Metformin (1 or 10 mg/kg orally, once a day for 40 consecutive days); 4: Stress group; groups 5 and 6: Stress + metformin (1 or 10 mg/kg, orally, once a day for 40 consecutive days). As gavage was stressful, the stress of gavage itself in the control group was alleviated by administering distilled water via gavage, and this was consistent across all groups.
3.3. Stress Model
Chronic variable stress (CVS) protocols were conducted based on other stress models with some modifications (21, 22). In brief, the aged rats received only one type of stress daily. The execution time varied during the day to minimize predictability. The protocol of stressors was divided into 7 types: (1) immobility (restraint) from 1 to 3 hours, (2) flashing lights (120 to 210 min), (3) isolation (2 to 3 days), (4) 45° angle inclination of the home cages for 4 - 6 hours, (5) damp bedding (300 mL water spilled onto bedding during 1.5 to 2 h), (6) cold exposure (consisting of placing rats individually into plastic cages and putting them in a cold room, 4°C for 1.5 to 2 h), and (7) tail pinched for 5 minutes (using a plastic clothespin at 8 cm distance from the tip of the tail). The stress process was performed for 40 days, as described in Table 1.
| Day of Treatment | Stressor Applied |
|---|---|
| 1 | Inclination of home cages (4 h) |
| 2 | Flashing light (120 min) |
| 3 | Tail pinched |
| 4 | Cold exposure (1.5 h) |
| 5 | Immobilization (2 h) |
| 6 | Damp bedding (1.5 h) |
| 7 | Isolation |
| 8 | Isolation |
| 9 | Isolation |
| 10 | No stressor |
| 11 | Inclination of home cages (6 h) |
| 12 | Immobilization (3 h) |
| 13 | Tail pinched |
| 14 | Damp bedding (2 h) |
| 15 | Cold exposure (1.5 h) |
| 16 | Flashing light (210 min) |
| 17 | Tail pinched |
| 18 | Isolation |
| 19 | Isolation |
| 20 | Isolation |
| 21 | Inclination of home cages (5 h) |
| 22 | Immobilization (2 h) |
| 23 | Cold exposure (2 h) |
| 24 | Tail pinched |
| 25 | Damp bedding (1.5 h) |
| 26 | Flashing light (180 min) |
| 27 | Immobilization (1 h) |
| 28 | Cold exposure (1.5 h) |
| 29 | Inclination of home cages (6 h) |
| 30 | No stressor applied |
| 31 | Isolation |
| 32 | Isolation |
| 33 | Tail pinched |
| 34 | Damp bedding (2 h) |
| 35 | Immobilization (3 h) |
| 36 | Cold exposure (2 h) |
| 37 | Inclination of home cages (4 h) |
| 38 | Flashing light (210 min) |
| 39 | Tail pinched |
| 40 | Immobilization (2 h) |
Schedule of Stressors Applied in This Study
3.4. Behavioral Tests
All neurobehavioral tests, including the elevated plus maze, Y‐maze, open field test, rotarod, exhaustive swimming exercise, forced swim tests, and Morris water maze, were started 24 hours after the last session of the chronic stress induction and on different days. All members of each group underwent all behavioral tests.
3.4.1. Anxiety-Like Behavior Assessment
The elevated plus maze was used to measure anxiety-like behavior. This device consisted of four arms as a plural sign (20). There were two closed arms and two open arms of the same size [50 × 10 cm], connected by a central square platform (10 × 10 cm). The test device was located at a height of approximately 50 cm from the ground. The animals moved for 5 minutes completely freely in all arms of the maze. A video system recorded the movements in the maze. Anxiety-like behavior was measured by two types of movement behavior: The percentage of time spent in the open arms (expressed as OAT%) and the percentage of entry into the open arms (abbreviated as OAE%).
[OAT% (the ratio of times spent in the open arms to total time spent in any arms × 100)]
[OAE% (the ratio of entries into open arms to the total entries × 100)].
3.4.2. Open Field Test
The Open-field Test (OFT) (a 50×50×50 cm chamber whose walls prevent escape) is a common measurement for anxiety. Rats were placed in the chamber's center and freely explored for 5 min. The activity of the animals was recorded and analyzed using the Ethovision software package (version 7.1, The Netherlands). The following parameters were reported: Distance moved (cm), velocity (cm/s), and central time (s) (23).
3.4.3. Working Memory Assessment
The Y‐maze apparatus was used to evaluate the working memory of aged rats. This device consisted of three symmetrical arms with an angle of 120 degrees (35 cm length × 5 cm width × 15 cm height). At the beginning of the test, each rat was placed in the maze's center. The movement of each rat was monitored spontaneously for 8 minutes. A complete rotation was defined as motion in all three arms (without returning to the repetitive arm). The rotation rate was the ratio of the number of rotations to the total number of complete rotations (20).
3.4.4. Measurement of Spatial Memory
The Morris Water Maze (MWM) test was chosen to measure the spatial memory of aged rats. The MWM had a black circular tank (60 cm in height and 140 cm in diameter). The tank of this test was full of water (22 + 1°C) and divided into four conceptual parts. In one of the centers of this quarter (target quarter), there was a black platform (diameter 10 cm) approximately 2 cm below the water level. We released each animal facing the wall of the tank in the water. The hidden platform came out of the water while checking the memory. Swimming time, swimming speed, and time spent in the target quadrant were examined with the automated video tracking system (Ethovision software; version 7.1, Noldos IT, The Netherlands) (24).
3.4.5. Evaluation of Physical Strength
Exhaustive swimming exercise was applied to examine the physical strength of aged animals. Each rat was allocated individually in an acrylic plastic pool (50 × 50 × 40 cm) filled with freshwater (30 cm deep, 25 ± 1°C). The water temperature of this pool was kept at 25°C. A steel ring equivalent to 5% of the body weight was attached to the tail. Fatigue, loss of coordination, and failure to return to the surface within 7 seconds were measured, and then fatigue time was recorded immediately (20). Finally, we took the animals out of the water and dried them with a towel.
3.4.6. Rotarod Treadmill Test
A Rotarod treadmill was used to evaluate the balance and coordination between the limbs of aged animals. The device consisted of a wheel whose rotational speed was variable (0 - 40 rpm) (25). The wheel was 20 cm away from the ground and was divided into 4 separate parts by spherical plates. For better learning of this test, each rat was trained twice for 3 minutes each time. Then, the duration of balance and movement of the animal on the rotating bar was checked 3 times (5 minutes for each trial) at an interval of 15 minutes. The average rotation time was recorded in seconds.
3.4.7. Depressive-Like Behavior Assessment
Depressive-like behavior was measured by the forced swim test (24). Briefly, each animal was placed separately in a Plexiglas cylinder (25 cm in diameter and 50 cm in height) containing water up to 35 cm in height at a temperature of 23 to 25°C. A camera recorded the animal's behavior for 6 minutes. Immobility time was measured during the last 5 minutes and considered a measure of depressive-like behavior. Immobility means that the animal did not move in different directions.
3.5. Measuring Body, Brain, and Adrenal Gland Weight
Body weight was measured since chronic stress could lead to significant weight loss. One day after the end of the behavioral experiments, the rats were sacrificed, and the brain and adrenal gland tissue weight was measured (22).
3.6. Blood Glucose Measurement
After 40 days of stress induction, blood samples were taken by scratching the animals' tails, and then an EasyGluco device made in Korea measured blood glucose.
3.7. Evaluation of Brain BDNF Protein and SOD Levels
After completing behavioral tests, animals were sacrificed under deep anesthesia by exposure to a CO2 atmosphere. The brain was rapidly removed from the skull and placed on a glass petri dish filled with ice-cold saline, and then the right hemisphere of the brain was isolated. Finally, the brain tissue was homogenized in ice-cold lysis buffer (containing 10 mM Tris–HCl, 1 mM EDTA, 0.1% SDS, 0.1% Na deoxycholate, 1% NP-40; 2 μg each of the protease inhibitors leupeptin, aprotinin, and pepstatin A; and 0.5 μmol/L PMSF pH 7.4). The lysate was centrifuged at 14,000 rpm for 20 minutes at 4°C. The supernatant was transferred to fresh tubes as the whole-cell fraction. The concentration of protein in each fraction was measured by the Bradford method. The supernatant samples were stored at -80°C until molecular investigations. The level of BDNF protein expression was measured using the ELISA method. A biochemical assay kit evaluated the SOD enzyme activity. Levels of BDNF protein expression and SOD enzyme activity in each experimental group were measured via different commercial assay kits (ZellBio, Germany).
3.8. Statistical Analysis
Data were analyzed using GraphPad Prism version 6 software package. All data were represented as Mean ± S.E.M. We used a one-way analysis of variance (ANOVA) followed by a Tukey post-hoc test for group comparison. P < 0.05 was considered statistically significant.
4. Results
4.1. Effects of Metformin and Stress on Body Weight, Brain Weight, Adrenal Gland Weight, and Blood Sugar
At the end of the study (40 days after the beginning of stress induction), weights (body, brain, adrenal gland) were calculated and shown in Table 2. The body weight of rats in the stress group decreased compared with the control animals, but it was insignificant (P > 0.05). The body weight of the stress + metformin 10 mg/kg group was significantly reduced compared with the control group (P < 0.05). According to Table 2, the mean brain weight in the stress group significantly decreased compared to the control group (P < 0.01). Moreover, as shown in Table 2, the adrenal gland weight of animals in the stress group (P < 0.001) and the stress + metformin group at a dose of 1 mg/kg (P < 0.01) increased significantly compared with the control group. In rats of the stress + metformin 10 mg/kg group (P < 0.01), adrenal gland weights were diminished compared with the stress group. There was no significant difference in blood sugar between the groups.
| Control | MET1 | MET10 | Stress | Stress + MET1 | Stress + MET10 | |
|---|---|---|---|---|---|---|
| Body weight (g) | 324.5 ± 9.27 | 325.37 ± 9.82 | 296.62 ± 13.01 | 290.57 ± 9.83 | 303.5 ± 10.88 | 279.5 ± 10.33 b |
| Brain weight (g) | 1.30 ± 0.02 | 1.29 ± 0.02 | 1.31 ± 0.01 | 1.20 ± 0.02 c | 1.25 ± 0.023 | 1.27 ± 0.017 |
| Adrenal weight (mg) | 44.19 ± 0.91 | 44.59 ± 0.92 | 44.12 ± 1.30 | 53.81 ± 1.24 d | 53.26 ± 1.77 d | 48.29 ± 1.41 e |
| Blood sugar (mg/dL) | 94.43 ± 2.92 | 95.50 ± 3.02 | 100.8 ± 2.73 | 96.71 ± 2.43 | 100.7 ± 2.23 | 99.29 ± 3.22 |
Body, Brain, and Adrenal Weights and Blood Sugar Changes a
4.2. Effects of Metformin and Stress on Anxiety-Like Behavior in the Elevated Plus Maze Test
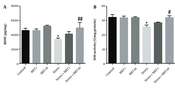
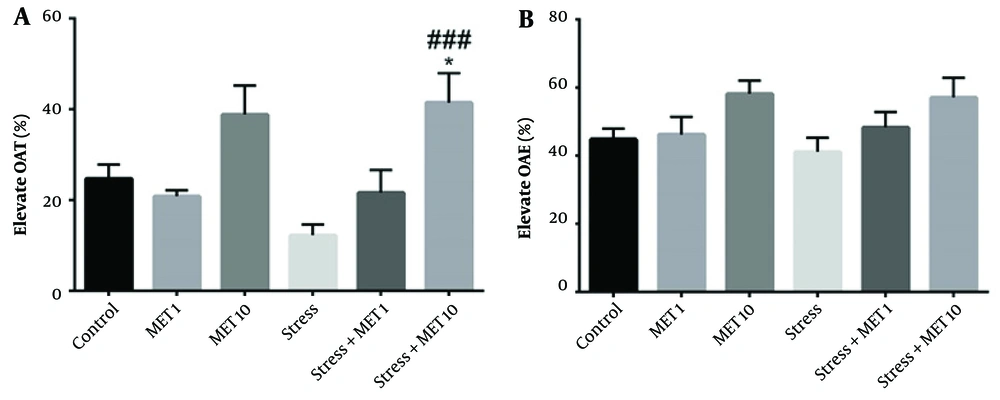
The results obtained from this test are shown in Figures 1A and B. Figure 1A shows the time spent in the open arm, indicating a significant increase in the stress + metformin 10 mg/kg group compared with the control (P < 0.05) and stress groups (P < 0.001), implying a decrease in anxiety in these groups.
Effect of chronic variable stress and metformin (MET) in the elevated plus maze test. Results are illustrated as mean ± S.E.M. One-way ANOVA followed by Tukey post hoc test was used to compare the values obtained from different experimental groups. *P < 0.05 vs. control, ###P < 0.001 vs. stress and control. OAT, open arm time; OAE, open arm entries.
4.3. Effects of Metformin and Stress on Anxiety in Open Field Test
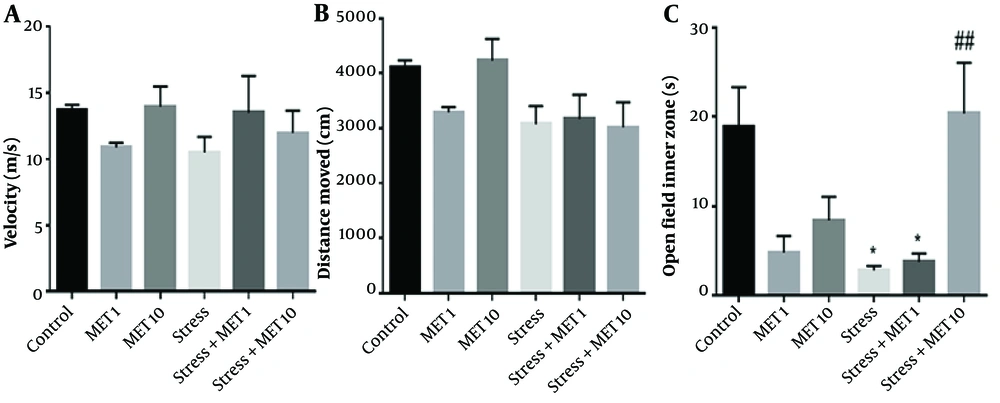
Figure 2A shows the speed of movement in different groups. There was no significant difference between the graphs, meaning that all groups were the same in terms of speed. Figure 2B also shows no difference in distance traveled between the groups. Moreover, as Figure 2C shows, the time spent indoors in the stress group and stress + metformin 1 mg/kg group significantly decreased compared with the control group, meaning an increase in anxiety behavior (P < 0.05). The stress + metformin 10 mg/kg group showed an enhancement compared with the stress group, which indicates a decrease in anxiety (P < 0.01).
4.4. Effects of Metformin and Stress on Muscle Fatigue by Exhaustive Swimming Exercise
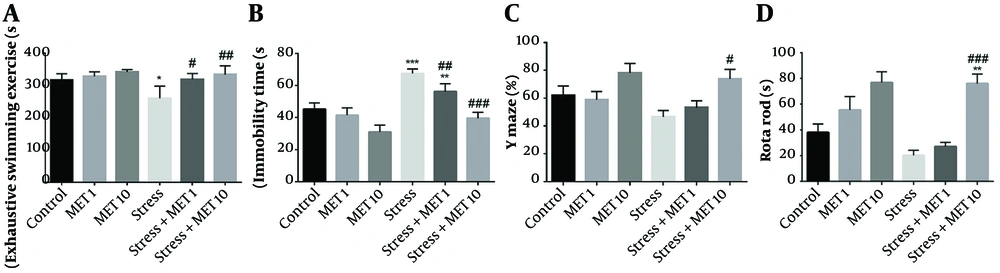
Figure 3A shows that the duration of muscle fatigue in the stress group was significantly reduced compared with the control group, meaning that muscle strength was reduced (P < 0.05). The stress + metformin 1 mg/kg (P < 0.05) and 10 mg/kg (P < 0.01) groups showed a significant increase in the duration of muscle fatigue compared with the stress group.
Effect of chronic variable stress and metformin (MET) on muscle fatigue, depression, working memory, and nerve-muscle balance. Results are illustrated as mean ± S.E.M. One-way ANOVA followed by Tukey post hoc test was used to compare the values obtained from different experimental groups. *P < 0.05, **P < 0.01, and ***P < 0.001 vs. control; #P < 0.05, ##P < 0.01, ###P < 0.001 vs. stress.
4.5. Effects of Metformin and Stress on Depression by Forced Swimming Test
Figure 3B exhibits that the stress (P < 0.001) and stress + metformin 1 mg/kg (P < 0.01) groups showed a significant increase in the immobility time and depression compared with the control group. However, the stress + metformin 10 mg/kg group had reduced immobility time to the level of the control group, which was statistically significant (P < 0.001) compared with stress animals. The metformin 1 mg/kg group (P < 0.01) also showed a significant decrease in the immobility time compared with the stress group but not as potent as the 10 mg/kg dose.
4.6. Effects of Metformin and Stress on Working Memory in the Y-maze Test
The working memory results are presented in Figure 3C. According to this figure, the stress + metformin 10 mg/kg (P < 0.05) group showed a significant increase in working memory compared with the stress group.
4.7. Effects of Metformin and Stress on Nerve-Muscle Balance by Rotarod
Figure 3D shows that the stress + metformin 10 mg/kg group had a significant increase compared with the control group (P < 0.01). Stress + metformin 10 mg/kg also had a significant increase compared with the stress group (P < 0.001).
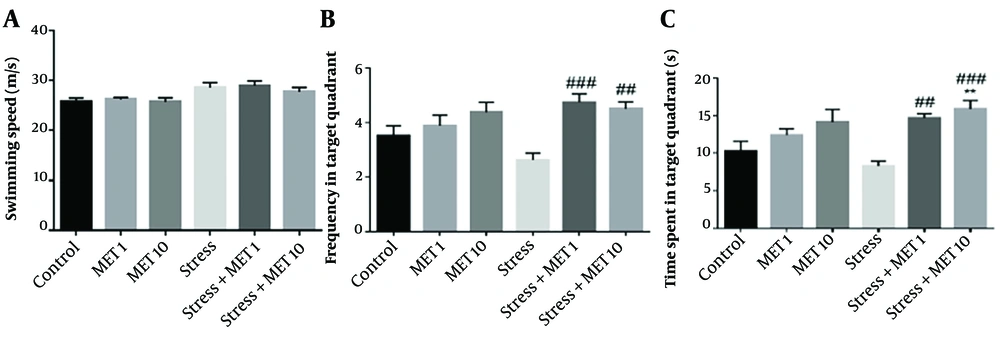
4.8. Effects of Metformin and Stress on Spatial Memory in Morris Water Maze
After 40 days of stress induction, the spatial memory was examined from several aspects by MWM. Figure 4A shows the swimming ability and the speed of movement, which are insignificant between the groups. In Figure 4B, the stress group showed a diminished frequency level in the target quadrant compared with the control group, but it was insignificant. However, treatment with metformin with both doses could significantly enhance this parameter compared with stress animals. Figure 4C shows the time spent in the target quadrant. This result exhibited a significant increase in the stress + metformin group of 10 mg/kg compared with the control group (P < 0.01). Furthermore, stress + metformin 1 mg/kg (P < 0.01) and 10 mg/kg (P < 0.001) groups showed a significant increase compared with the stress group.
Effect of chronic variable stress and metformin (MET) on Morris water maze. Results are illustrated as mean ± S.E.M. One-way ANOVA followed by Tukey post hoc test was used to compare the values obtained from different experimental groups. B: ##P < 0.01 and ###P < 0.0001 vs. stress, C: **P < 0.01 vs. control ##P < 0.01 and ###P < 0.001 vs. stress.
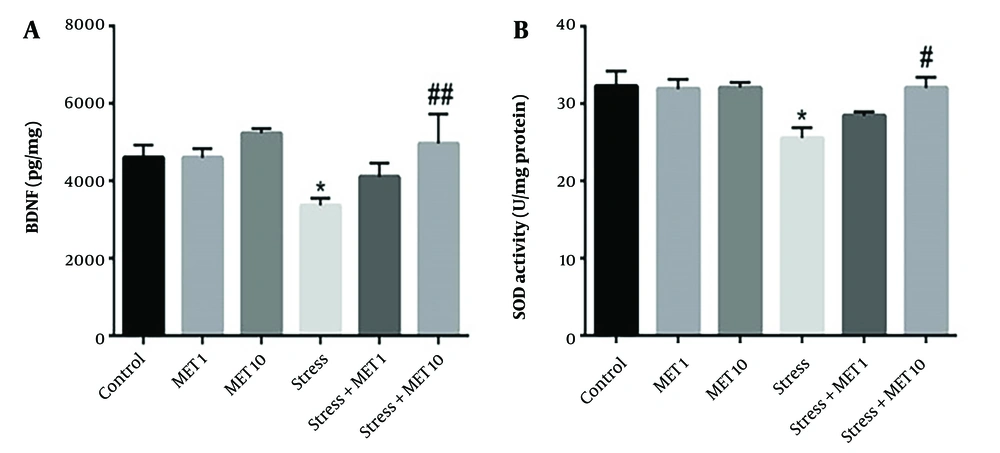
4.9. Effects of Metformin and Stress on BDNF
Brain tissue BDNF level is presented in Figure 5A. Induction of chronic variable stress caused a significant decrease in this protein compared with the control group (P < 0.05). The stress group + metformin 10 mg/kg showed a significant improvement in the level of BDNF (P < 0.01) compared with the stress group.
Effect of chronic variable stress and metformin (MET) on level of BDNF and SOD. Results are illustrated as mean ± S.E.M. One-way ANOVA followed by Tukey post hoc test was used to compare the values obtained from different experimental groups. *P < 0.05 vs. control; #P < 0.05 and ##P < 0.01 vs. stress.
4.10. Effects of Metformin and Stress on SOD
The results of measuring the activity of the SOD enzyme are shown in Figure 5B. A significant decrease in this enzyme's activity level was observed in the stress group compared with the control group (P < 0.05). The stress + metformin 10 mg/kg group showed a significant increase and improvement in the level of this enzyme compared with the stress group (P < 0.05).
5. Discussion
The present study was designed to evaluate the neurobehavioral effects of the aging process and to investigate the potential protective effects of metformin treatment on aged animals under stress conditions. Cognitive functions, memory, and physical abilities decline during aging, and animals develop depression. The results of this study show that the stress protocol exacerbates the damage caused by aging. However, oral chronic administration of metformin prevents the detrimental effects of aging and stress, such as memory dysfunction, lack of muscle strength, anxiety, and depression in aged male rats.
Throughout the aging phase of human life, neurological disorders appear due to cognitive and synaptic function reduction in some areas of the brain (26). Aging is parallel with gradual memory impairment (27). Lopez-Otin et al. showed that ROS plays an important role in cell signaling and survival but can cause cell damage at chronically high levels (2). The aging process and CVS are accompanied by ROS generation, leading to pathological changes, including cell death or memory loss (28). In our study, memory loss was observed in the group of aged animals under stress, and probably, the high ROS rate could be considered one reason for this change.
The study by Hjortskov et al. showed that high blood pressure could be a complication of daily stress (29). Another study showed a decrease in the brain weight of animals with high blood pressure compared with healthy animals (30). These findings could justify our observation of brain weight reduction in stressed rats compared with the control group. Furthermore, aging and chronic stress cause cellular apoptosis and death in different brain parts (31).
Anxiety disorders account for many psychiatric disorders in the elderly (32). Depression in old age could be a lifelong recurrent mental illness. Studies have also shown a strong association between depression and diseases associated with aging, such as Parkinson's disease and Alzheimer's disease (33). Stressful situations are linked with a higher rate of mental disorders (12). Stress can be attributed to stress-related illnesses such as depression due to impaired activity of enzymes, including SOD and catalase (34). In our study, we found increased levels of anxiety and depression in aged stressed rats. In our study, the stress group consisted of aged animals under CVS, meaning that aging and stress together may lead to more complications in different aspects.
Like ours, Tagliari et al. showed that CVS in rats resulted in body weight loss, adrenal gland weight gain, and increased depression symptoms. They recorded increased levels of inflammatory cytokines such as IL-1β, IL-6, and TNF-α by inducing stress. They revealed inflammation and cholinergic dysfunction as possible causes of neurological dysfunction in some depressed patients (22). You et al. showed that in mild chronic stress, BDNF mRNA expression decreases due to stress induction (35). In the aging process, BDNF and its receptors are impaired (36). Herein, our results confirm the association between stress and anxiety, and we observed a decrease in BDNF levels in aged and stressed animals compared with aged animals.
Metformin, a common drug for type 2 diabetes mellitus, plays an important role in mechanisms involved in aging. Metformin has different effects on the aging process, including ROS reduction and activation of AMPK (37). In addition to controlling blood sugar, studies show that metformin has beneficial effects on physical function (such as movement), weight, cardiovascular system, and mental health (depression and cognitive impairment) (38). Metformin exhibited protection against depression in elderly diabetic patients (39). This study, in line with the previous studies, showed that metformin has beneficial effects on depression in aged animals.
A study by Sarkaki et al. showed the neuroprotective effects of metformin on memory enhancement in the elevated plus maze and open field tests (40). Wang et al. published an article stating that metformin causes neurogenesis and increases learning and memory in the MWM test (41). Our study also showed that metformin exerts protective effects on the nervous system and improves memory in elderly rats with chronic stress conditions.
Researchers investigating the possible effects of metformin on methotrexate adverse cognitive events revealed that metformin improves the number of immature neurons and the number of proliferating cells and their survival in the subgranular zone of the hippocampal dentate gyrus, which well indicates the protective effect of metformin on neurogenesis; they also reported a memory improvement (42). In addition, in a recently published study, metformin was introduced as a drug that can delay the aging of stem cells, increase their lifespan, and even cause rejuvenation in oligodendrocyte progenitor cells (43). It seems that metformin, as a mysterious drug, can regenerate the structure of the human body.
Metformin increased the levels of BDNF and improved neuronal status and memory by increasing AMKP in the model of cerebral forebrain ischemia (44). Metformin could enhance brain BDNF levels in d-galactose-induced aging in ovariectomized mice and reduced anxiety in the elevated plus-maze test (45). Our results showed increased BDNF levels in stress-aged animals accompanied by metformin administration. Fatemi et al. showed that metformin increases SOD activity in aged animals compared to those without metformin (46). Our study also showed that chronic treatment with metformin elevates the SOD activity in stress-aged animals. The study by Zakeri et al. utilized exhaustive swimming exercises and found that metformin could reduce sarcopenia in the aging model (20) and observed a similar protective mechanism.
5.1. Conclusions
Our study suggests that metformin at a dose of 10 mg/kg could improve aging-related dysfunctions (memory impairment, anxiety, and decreased physical potency) in animals under the protocol of chronic variable stress. The possible mechanism of this action is related to the antioxidant and neuroprotective activities via increased SOD and BDNF.