1. Background
Multiple Sclerosis (MS) is the most frequent non-traumatic neurological disability that affects young adults (1). It is becoming more common and widespread in developed and developing countries (2, 3). Its prevalence is much higher in women and people between 20 and 40 years old (4). Multiple genes (5, 6) and various well-defined environmental factors, such as vitamin D (7-9) or ultraviolet light exposure (10-12), smoking (13-15), obesity, and Epstein-Barr virus infection, potentially contribute to susceptibility to this multifactorial disease (16).
Infectious organisms, most likely viruses, have been suspected of activating the autoimmune response in MS patients with a genetic predisposition to the disease. Even though various infectious microorganisms have been studied, no organism has been identified as a confirmed cause (17, 18). The potential role of infectious microorganisms, including retroviruses, in the pathogenesis of MS has been an area of investigation in MS research. While the exact cause of MS remains unknown, it is believed to involve a combination of genetic and environmental factors, including potential infectious triggers. However, the involvement of retroviruses in MS pathogenesis remains a subject of debate and ongoing research.
Increasing evidence demonstrates that human retroviruses may have a role in the etiology of several acute and chronic neurological disorders. One specific retrovirus studied concerning MS is the human endogenous retrovirus (HERV). The HERVs are remnants of ancient retroviral infections that have become part of the human genome. Some studies have reported increased expression of HERV-related elements in MS patients, particularly in active MS lesions. It has been hypothesized that the expression of HERV elements could lead to inflammation and immune dysregulation, contributing to MS pathogenesis. However, further research is needed to fully understand the significance and mechanisms of HERV involvement in MS. Human Immunodeficiency Virus (HIV), which is the main cause of acquired immunodeficiency syndrome (AIDS), was found in the brain and isolated from neural tissue and cerebrospinal fluid of patients with encephalopathy related to AIDS (19-22). Furthermore, Human T-Lymphotropic Virus Type 1 (HTLV-I) is involved in the pathogenesis of Tropical Spastic Paraparesis (TSP), a myelopathy of uncertain origin that is common in tropical areas where the virus is endemic. HTLV-I is also associated with adult T-cell leukemia (ATL) (23-25). The HTLV-1 virus is endemic in different parts of the world, including Khorasan Province in northeast Iran (26, 27), and there are similarities between the symptoms of patients with this virus and the symptoms of MS (28, 29). Several observations show that the transmission route of HTLV is similar to other retrovirus, such as HIV (30, 31).
Both MS and HIV infection are examples of immune diseases where there is a change in the inflammatory response of CD4+ T and CD8+ T lymphocytes (32). It has been observed that HIV-positive patients have a lower risk of developing MS. Furthermore, patients who undergo antiretroviral therapy have reported a reduction in MS symptoms (33-35). According to Gessain et al., approximately 60% of TSP patients showed antibodies to HTLV-I, while 4% of the control group had such antibodies (23). Another study by Koprowski et al. revealed that Swedish and American patients with MS had antibodies in their sera and cerebrospinal fluids that cross-reacted with HIV or HTLV-I proteins and that four out of eight patients' cerebrospinal fluid cells had HTLV-I-related RNA sequences (36). In a patient with MS who was infected with HIV, antiretroviral therapy improved her MS symptoms (37).
Besides retroviruses, other infectious agents have been investigated as potential triggers for MS or contributors to disease progression. For example, Epstein-Barr virus (EBV), a member of the herpesvirus family, has been extensively studied concerning MS. Epidemiological studies have shown an association between prior EBV infection and an increased risk of developing MS.
2. Objectives
Due to the scarcity of studies examining the connection between MS and HTLV-I/II and HIV infection, alongside contradictory findings on this matter, we decided to conduct a research study to explore the presence of HTLV-I/II and HIV antibodies in the sera of MS patients residing in northeastern Iran.
3. Methods
3.1. Study Design and Population
This case-control study was performed on patients with MS from January 2018 to January 2019 in the Neurology Department of Ghaem Hospital, affiliated with Mashhad University of Medical Sciences, Mashhad, Iran.
The case group comprised serum samples from 38 MS patients previously diagnosed by a neurologist. All patients diagnosed with MS who provided written consent to participate in the study were included during the enrollment period at the neurology clinic. The diagnosis of MS typically involves a combination of clinical evaluation, medical history assessment, neurological examination such as MRI, and various diagnostic tests. The process aims to rule out other conditions with similar symptoms and establish the presence of characteristic features suggestive of MS. The diagnosis of MS was based on McDonald's criteria, which incorporate clinical, imaging, and laboratory findings to enhance the accuracy and efficiency. In addition, the study recruited 41 individuals from different hospital wards who did not have symptoms of MS as a control group. Patients' demographic and medical information was collected using a relevant checklist and medical records. We performed frequency matching in the present study to ensure that the distribution of certain variables is the same among cases and controls. All serum samples were analyzed using ELISA kits for HIV-I/II and HTLV-I/II antibodies.
3.2. Data Analyses
The data were analyzed by IBM SPSS version 20.0 software (SPSS Inc., Chicago, IL, USA). Initially, the Kolmogorov-Smirnov test was used to determine the data's normal distribution. Categorical variables were described as frequencies (%), and continuous variables were described as mean ± Standard Deviation (SD). Student t-test was used in data analysis. The Chi-square test was employed to evaluate qualitative variables, and Fisher's exact test was utilized when more than 20% of the predicted frequencies of the tables were less than 5. A p-value of less than 0.05 was considered statistically significant.
3.3. Ethical Consideration
The patients completed informed consent forms. Moreover, the Ethics Committee of Mashhad University of Medical Sciences fully approved the study protocol per the Helsinki Declaration guidelines (#IR.MUMS.fm.REC.1396.455).
4. Results
A total of 79 individuals were investigated, including 38 MS patients and 41 healthy individuals who served as the case and control groups, respectively. The mean age of the individuals in the case and control groups was 33.8 ± 8.9 and 32.1 ± 3.0 years, respectively. Table 1 illustrates the patients' demographic characteristics, demonstrating no statistically significant differences regarding age and gender between the case and control groups (P: 0.265 and P: 0.305, respectively).
| Variables | Case Group | Control Group | P-Value |
|---|---|---|---|
| Age, y (mean ± SD) | 33.8 ± 8.9 | 32.1 ± 3.0 | 0.265 |
| Gender | 0.305 | ||
| Male | 9 (23.7) | 14 (34.1) | |
| Female | 29 (76.3) | 27 (65.9) | |
| Occupation | 0.034 | ||
| Employed | 13 (34.2) | 25 (61) | |
| Housekeeper | 23 (60.5) | 14 (34.1) | |
| Student | 2 (5.3) | 2 (4.9) |
a Values are expressed as No. (%) unless otherwise indicated.
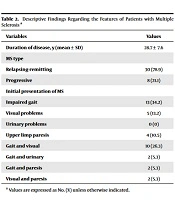
Regarding MS types, most patients (78.9%) had relapsing-remitting MS, whereas 21.1% had progressive MS. The case group included 38 patients who had experienced MS for a minimum of 14 years and a maximum of 44 years. The most common initial symptom of MS was impaired gait, which accounted for 34.2%, followed by gait and visual impairment, which accounted for 26.3% of the cases (Table 2).
| Variables | Values |
|---|---|
| Duration of disease, y (mean ± SD) | 28.7 ± 7.6 |
| MS type | |
| Relapsing-remitting | 30 (78.9) |
| Progressive | 8 (21.1) |
| Initial presentation of MS | |
| Impaired gait | 13 (34.2) |
| Visual problems | 5 (13.2) |
| Urinary problems | 0 (0) |
| Upper limp paresis | 4 (10.5) |
| Gait and visual | 10 (26.3) |
| Gait and urinary | 2 (5.3) |
| Gait and paresis | 2 (5.3) |
| Visual and paresis | 2 (5.3) |
a Values are expressed as No. (%) unless otherwise indicated.
As shown in Table 3, the frequency of HIV-I/II antibodies was 0.0% in the case group and 0.0% in the control group, which was not significantly different (P: 0.999). Besides, the prevalence of HTLV-I/II antibodies was 0.0% in the MS group and 2.4% in the control group, with no statistically significant difference between the two groups (P: 0.999).
| Variables | Case Group, No. (%) | Control Group, No. (%) | P-Value |
|---|---|---|---|
| HIV-I/II antibody | 0.999 | ||
| Positive | 0 (0) | 0 (0) | |
| Negative | 38 (100) | 41 (100) | |
| HTLV-I/II antibody | 0.999 | ||
| Positive | 0 (0) | 1 (2.4) | |
| Negative | 38 (100) | 40 (97.6) |
5. Discussion
This study aimed to evaluate the presence of HTLV-I/II and HIV antibodies in the sera of patients with MS. Our results demonstrated no significant differences between MS patients and controls regarding the mentioned antibodies.
In 1989, Reddy et al. first investigated the correlation between the HTLV-I virus and MS (29). They performed a comparative analysis involving six MS patients and twenty healthy individuals. Through DNA blot analysis and molecular cloning of amplified DNAs, they discovered HTLV-I sequences in all six MS patients and one individual from the control group (P = 0.01). The researchers concluded a correlation between the HTLV-I virus and MS patients. However, further studies are needed to understand the underlying cause (29). The findings of our investigation are in direct contradiction to those of Reddy et al. The notable discrepancy is likely attributed to the small sample size in their study and the differences in our method for assessing HTLV-I antibodies compared to Reddy et al.'s approach (29). In another study, Gold et al. conducted a larger cohort study to assess the correlation between MS and HIV infection, with a case group comprising 21,207 HIV-positive individuals (33). All of these individuals were followed for around 6 years to assess the incidence of MS in both groups. The incidence ratio of developing MS in HIV-positive patients, relative to the control group, was 0.38 (95% confidence interval = 0.15 - 0.79). Their result revealed a significant association between HIV infection and a reduced chance of developing MS (33).
Some studies are in line with our results. A study by Watanabe et al. examined the presence of HTLV-I DNA integrated into peripheral blood mononuclear cell DNA of nine MS patients by polymerase chain reaction, which revealed that all of these patients were negative and there was no HTLV-I infection in patients with MS (38). In our study, the HTLV-I/II antibody was negative in all MS patients. A large-scale cohort study was conducted on 5,018 HIV-positive patients and 50,149 HIV-negative people. For about 6.5 years, all of the individuals in this research were monitored for MS. Finally, the MS incidence ratio among HIV patients was found to be 0.3 (95% confidence interval = 0.04 - 2.2), although this rate was not statistically significant (34). Schneider et al. utilized ELISA techniques to look for HTLV-I/II and HIV antibodies in 18 cerebrospinal fluid and 135 serum samples from MS patients (28). In the ELISA test for HTLV-I, none of the serum or cerebrospinal fluid samples responded to HIV antigens, and only 3 out of 135 serum samples, but no MS CSF, exhibited increased reactions. Immunoprecipitation, on the other hand, classified these positive results as non-specific. As a result, no evidence of HTLV-I or HIV infection, as well as related retroviruses, was identified in MS patients (28). These findings are consistent with our investigation, which found that HTLV-I/II and HIV antibodies in all MS patients were negative.
The relationship between HIV and MS has been an area of interest in medical research. While both are distinct diseases with different underlying mechanisms, studies have explored potential associations and interactions between them. Both HIV and MS involve the immune system in different ways. For example, HIV is primarily known for its ability to suppress the immune system, specifically targeting CD4+ T cells (39). Consequently, lower immune system activation can lead to a lower manifestation of MS as an autoimmune disease. Also, HIV infection can lead to various neurological complications, including cognitive impairment, peripheral neuropathy, and opportunistic infections affecting the central nervous system. These complications may mimic MS symptoms or be misdiagnosed as MS, highlighting the importance of accurate diagnosis and differentiation between the two conditions. Furthermore, several studies have indicated the presence of shared risk factors between HIV and MS, including genetic susceptibility and environmental factors. However, the absence of HIV antibodies in MS patients suggests that active HIV infection is not a major factor contributing to the development of MS (40). The evidence supporting a direct causal link between the two diseases remains limited and requires further investigation. This finding supports the understanding that MS is primarily an autoimmune disease rather than a consequence of HIV infection.
Some studies have explored the possible involvement of HTLV-I/II in MS, given their ability to infect T cells and cause immune dysregulation. However, the absence of HTLV-I/II antibodies in MS patients suggests that these retroviruses are unlikely to play a significant role in the pathogenesis of MS. This finding indicates that the mechanisms underlying MS development may differ from those associated with HTLV-I/II-associated diseases.
It is important to note that while the absence of HTLV-I/II and HIV antibodies in MS patients provides valuable insights, it does not completely rule out the possibility of other viral infections or viral triggers playing a role in MS pathogenesis. The complex interplay between genetic, environmental, and immunological factors in MS requires further research to fully understand the disease's underlying mechanisms.
5.1. Limitations and Future Directions
Many studies exploring the association between MS and infectious agents like HTLV or HIV are often observational studies, such as case-control studies or retrospective analyses. While these studies can provide valuable insights, they have inherent limitations, including recall bias, selection bias, and the inability to establish causality. The other one is small sample sizes, which can limit the statistical power to detect subtle associations or differences. Additionally, sample selection bias may arise due to recruiting patients from specific populations, such as specialized clinics or specific geographic regions, which may not represent the broader MS population. Studies exploring infectious agents' relationship with MS must carefully account for confounding factors. Factors such as genetic susceptibility, environmental exposures, and other coexisting infections may influence the outcomes and complicate the interpretation of study findings. Thus, further research with larger sample sizes, rigorous study designs, and comprehensive consideration of confounding factors is necessary to better understand the potential involvement of infectious agents in MS pathogenesis.
5.2. Conclusions
According to the findings, this study could not specify a relationship between the presence of HTLV-I/II or HIV antibodies, and MS. Further research can clarify the mechanisms by which infectious agents may influence MS development and progression. Multiple sclerosis is a heterogeneous disease with various clinical subtypes and disease courses. It is likely that different infectious agents, or combinations thereof, may play a role in different subgroups of MS patients. Exploring the potential involvement of other infectious agents may help identify subtypes or specific patient populations that could benefit from targeted interventions. By expanding our knowledge in this area, we can better understand the complex interactions between infectious agents, genetic factors, and the immune system in MS, ultimately leading to improved diagnosis, treatment, and preventive strategies.