1. Context
Inborn errors of metabolism are presented as severe and rare groups of genetic disorders due to a defect in a metabolic pathway. The metabolic diseases include defects of glucose homeostasis, defects of amino acids, defects of fatty or organic acids, defects of lactate/pyruvate, defects of peroxisomes, and some other rare defects. Every child with unexplained neurological deterioration, metabolic acidosis, hypoglycemia, inappropriate ketosis, hypotonia, cardiomyopathy, encephalopathy, seizure, hepatocellular dysfunction, gastrointestinal signs, such as poor feeding, diarrhea, and vomiting, and failure to thrive should be suspected of having a metabolic disorder. They can cause ophthalmopathy or be associated with autism. Although metabolic disorders are rare, they might develop at any age, and the exact differential diagnosis must be considered. The differential diagnosis of metabolic disorders, such as adrenal insufficiency, sepsis, and congenital heart diseases, might be ruled out. The early diagnosis of metabolic disorders is the most complicated task for some clinicians because they need to know when to consider them and which tests to order for evaluation (1-3).
The deficiencies of functional enzymes cause defects in metabolic pathways and lead to aminoacidopathies (4). The accumulation of toxic substrates, intermediate metabolites produced from alternative metabolic pathways, and problems in energy production and utilization caused by a deficiency of products beyond a block lead to the consequences. Every metabolic disease has several forms that depend on the age of onset, clinical severity, and inheritance pattern. The first steps in metabolic therapy must be started as soon as possible to reduce precursor substrate load, provide caloric and fluid support, remove metabolites via dialysis, divert metabolites, and supplement with cofactor(s) (5, 6).
Amino acids, as the units of proteins, have numerous important functional and structural roles in the human body. They are the precursors of hemoglobin, hormones, enzymes, and neurotransmitters. The endogenous and dietary proteins are catabolized to amino acids, which might serve as energy sources. In catabolic processes, ammonia is eliminated through the urea cycle, and the carbon skeleton is replenished in the Krebs cycle. The quantitative analysis of amino acids has become an essential task in this regard, as they are important targets for identifying the metabolic profile of amino acid disorders (7).
The assessment of amino acids in body fluids is needed for the diagnosis and treatment of diseases. Although there have been advances in numerous methods of analysis of amino acids, such as chromatography (8-11) and electrophoresis (12-15), most methods still need extra procedures or have a lack of selectivity and sensitivity. Additionally, the evaluation of the amino acids in biological samples is difficult, mainly due to the presence of some matrices that interfere with the separation and detection procedure of amino acids. Therefore, faster, more selective, and more sensitive methodologies are required to analyze amino acids. The analytical techniques based on mass spectrometry, such as liquid chromatography with tandem mass spectrometry (LC-MS-MS), gas chromatography-mass spectrometry (GC-MS), and high-performance liquid chromatography (HPLC), and computational approaches to the interpretation of raw data help evaluate the measurement of amino acid levels in biological samples (8-10).
Mass spectrometry (MS) is a technique that is used to assess the mass-to-charge ratio of ions. Mass spectrometry is used in many different fields, such as proteins, peptides, and amino acids. Tandem mass spectrometry, also known as MS/MS or MS2 (Tandem mass spectrometry), is used to analyze biomolecules, such as proteins and peptides. The molecules of a biological sample are ionized and separated by their mass-to-charge ratio (often given as m/z or m/Q). Then, they are detected to make it possible to identify them in regular mass spectrometers.
Phenylketonuria (PKU), homocystinuria, tyrosinemia, non-ketotic hyperglycemia (NKH), and maple syrup urine disease are the most known disorders affecting amino acid metabolism. The aforementioned disorders are autosomal recessive and can be diagnosed by amino acid content analysis in body fluids (4).
As mentioned above, due to the overlapping symptoms of metabolic disorders, the diagnosis of aminoacidopathies is challenging for most clinicians. Recent advances in the diagnosis can help improve the prognosis of these disorders. As a result, this review summarizes the novel clinical manifestations and diagnostic methods of aminoacidopathies.
2. Evidence Acquisition
PubMed, Cochrane, Embase, and CINAHL were searched with MeSH terms: ‘inborn errors of metabolism' OR ‘Metabolism, Inborn Errors' (MeSH) AND ‘Humans' (MeSH) AND 'Amino Acids/therapeutic use'[MeSH] AND ‘Newborn, Child' (MeSH) OR ‘child' OR ‘newborn' AND "Neonatal Screening"[MeSH].
The present study summarizes the following issues:
(1) Clinical and laboratory diagnoses of PKU
(2) Clinical and laboratory diagnoses of tyrosinemia
(3) Clinical and laboratory diagnoses of methionine, homocysteine, and cysteine
(4) Clinical and laboratory diagnosis of sulfite oxidase deficiency and molybdenum cofactor deficiency
(5) Clinical and laboratory diagnoses of tryptophan
(6) Clinical and laboratory diagnoses of glycine
(7) Clinical and laboratory diagnoses of hyperoxaluria
(8) Clinical and laboratory diagnoses of creatine deficiency disorders
(9) Clinical and laboratory diagnoses of serine
(10) Clinical and laboratory diagnoses of proline
(11) Clinical and laboratory diagnoses of glutamine
(12) Clinical and laboratory diagnoses of urea cycle defect
2.1. Clinical and Laboratory Diagnoses of PKU
Phenylketonuria is the most known disorder caused by an inherited error in amino acid metabolism. The classic form is due to a mutation in the phenylalanine hydroxylase gene or cofactor deficiency (16, 17). This disease can be caused by two following reasons:
(1) Deficiency of the phenylalanine hydroxylase enzyme, which converts phenylalanine (Phe) to tyrosine (Tyr).
(2) Deficiency of tetrahydrobiopterin (BH4), which is a cofactor for the production of phenylalanine, serotonin, dopamine, and nitric oxide (18).
As a result, phenylalanine accumulates in the blood and causes block of the cerebral uptake of other large neutral amino acids, such as tyrosine and tryptophan, impairing brain protein synthesis. If the patient is undiagnosed and left untreated, this disorder can lead to severe mental retardation, epilepsy, behavioral problems, and phenotypic signs, such as a musty odor eczema, light pigmentation of the dermis, eyes, and hair, and cortical blindness (19). Early diagnosis and treatment are essential to avoid the severe effects and morbidity of the disease (20). Currently, due to the worldwide screening of infants, PKU is usually diagnosed in infancy. A drop of dried blood spot testing (DBS) sample is collected from the neonate’s heel for screening.
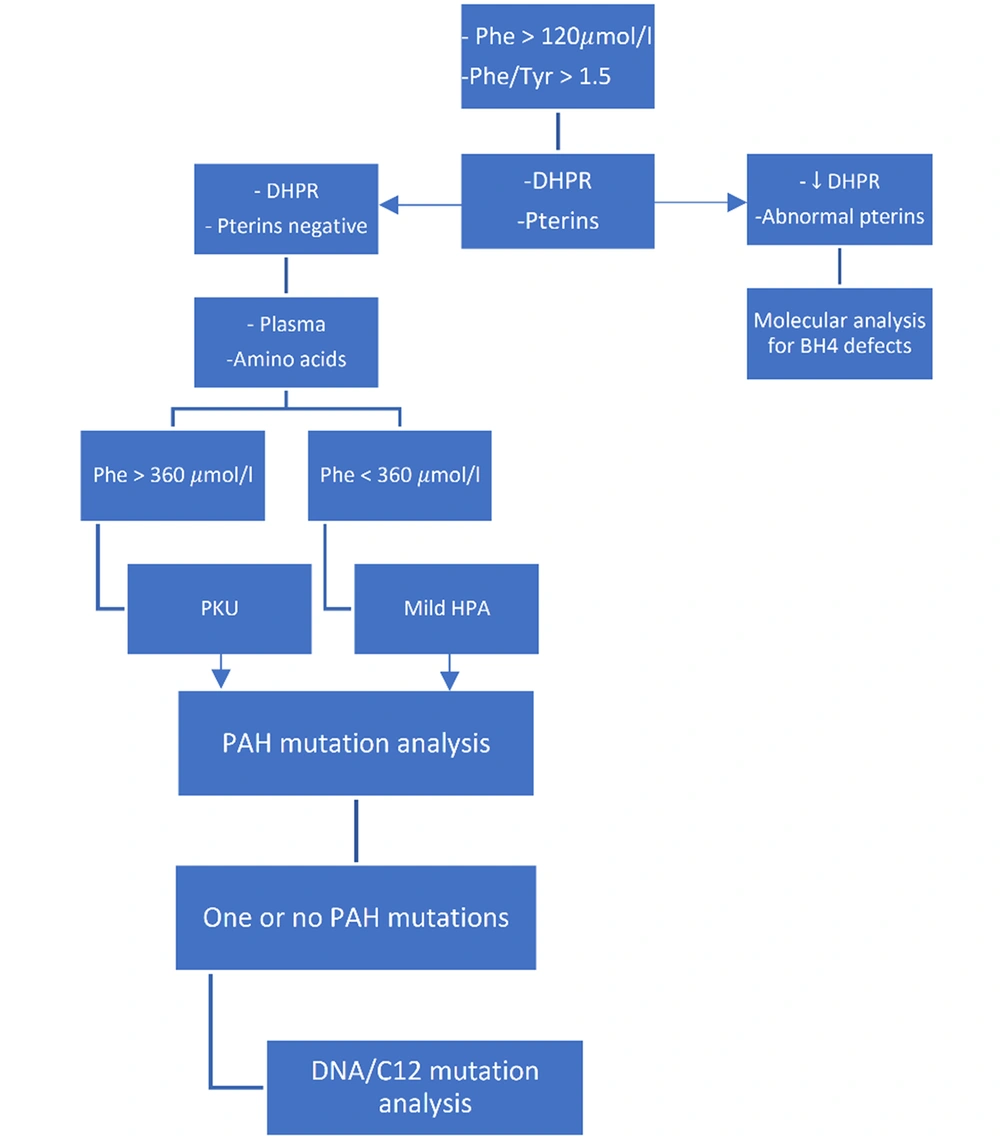
Bacterial inhibition assay (BIA), fluorometric microassay (FMA), and tandem mass spectrometry (MS/MS) techniques can be used to screen PKU. The first two methods identify only one amino acid; however, the TMS method measures several amino acids. Tandem mass spectrometry can simultaneously measure phenylalanine and tyrosine levels and is a more sensitive method for screening PKU. In the past, the cut-off of phenylalanine for a positive screening result was 240 µmol/L (4 mg/dL). However, in the TMS method, the positive screening result for PKU was determined by phenylalanine with a serum concentration of 120 µmol/L in combination with a phenylalanine-to-tyrosine ratio greater than 1.50 (19) (Figures 1 and 2).
Screening and diagnosis of phenylketonuria and monitoring treatment efficacy (19)
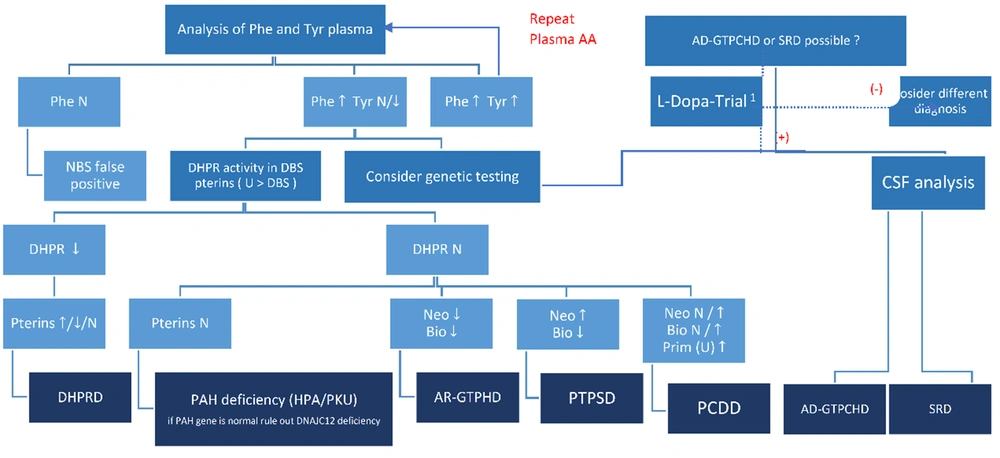
The diagnosis of hyperphenylalaninemia subtypes in the newborn screening. AD-GTPCHD, autosomal dominant guanosine triphosphate cyclohydrolase I deficiency; ARGTPCHD, autosomal recessive-guanosine triphosphate cyclohydrolase I deficiency; Bio, biopterin; DHPR, dihydropteridine reductase; DHPRD, dihydropteridine reductase deficiency; N, normal; NBS, newborn screening; Neo, neopterin; PAH, phenylalanine hydroxylase; Phe, phenylalanine; Tyr, tyrosine; Prim, primapterin; PTPSD, 6-pyruvoyltetrahydropterin synthase deficiency; SRD, sepiapterin reductase deficiency; PKU, phenylketonuria; U, urine; CSF, cerebrospinal fluid; DBS, dried blood spot testing; PCDD, pterin-4- alpha-carbinolamine reductase deficiency; (+), positive effect; (-), no or no apparent effect (21).
Six enzymes are involved in BH4 deficiency, which causes rare metabolic neurological disorders due to the insufficient synthesis of monoamine neurotransmitters, such as dopamine and serotonin, which are diagnosed by urine pterins (i.e., neopterin and biopterin). Furthermore, DBS and dihydropteridine reductase (DHPR) must be validated by genetic analysis.
Treatment depends on the types involved in the synthesis and recycling of BH4. The treatment is summarized as follows, although there are details in the consensus guidelines (22):
(1) BH4 or Kuvan (5 - 10 mg/kg)
(2) Carbidopa: This drug prevents the environmental conversion of L dopa to dopamine. The starting dose is 1 - 2 mg/kg (based on the dose of L-dopa) in four divided doses. Moreover, to prevent possible drug side effects, including nausea, vomiting, diarrhea, and restlessness, the dose is gradually increased to a maintenance dose of 5 - 10 mg/kg/d.
(3) 5-HT (5-hydroxytriptamine) with the same dose of L-dopa
(4) Folinic acid 15 mg/d
(5) Medications used to treat Parkinson’s disease reduce the symptoms of deficiency.
2.2. Clinical and Laboratory Diagnoses of Tyrosinemia
Most food we eat contains tyrosine which indirectly enters the Krebs cycle to produce energy during multiple-step processes. A group of genetic disorders, including tyrosinemia II, tyrosinemia type III, hawkinsinuria, alkaptonuria, and tyrosinemia type I, respectively, can be produced by disruptions in the multistep process that breaks down tyrosine.
The clinical presentations of tyrosinemia include tyrosinemia type I (hepatorenal) (23), tyrosinemia type II (oculocutaneous, rarely mild intellectual disability) (24), and tyrosinemia type III (neurological symptoms, e.g., mental retardation and neurodevelopmental delay (25)), hawkinsinuria (e.g., transient acidosis, ketosis, and hepatomegaly with average intelligence quotient [IQ]), and alkaptonuria (e.g., change in skin color, ochronosis, black spot in sclera, osteoarthritis, and kyphosis).
The diagnosis of tyrosinemia is made when there is an increase in plasma tyrosine and urine metabolite of tyrosine (succinyl acetone) (23) and phenylalanine (4-hydroxyphenylpyruvic acid [4-HPPA] and 4-hydroxyphenyllactic acid [4-HPLA]) in HPLC, MS/MS, and GC-MS methods, respectively. Different clinical manifestations and physical exams define different types of tyrosinemia as hepatorenal, ocular cutaneous, and neurologic combinations observed in types I, II, and III, respectively. However, an increase in succinyl acetone is specific to tyrosinemia type I and not observed in types II and III.
In hawkinsinuria, an increase in plasma tyrosine, 5-oxoproline, and urine (4-HPPA and 4-HPLA) was observed in HPLC, MS/MS, and GC-MS methods, respectively. Additionally, in alkaptonuria, increasing urine homogentisic acid by the GC-MS method is characteristic (Table 1).
| Number | Enzyme Name | Disease | Major Clinical Manifestations | Treatment |
|---|---|---|---|---|
| (A) | Tyrosine aminotransferase | Tyrosinemia type II | Corneal thickening, developmental delay, and hyperkeratosis of palms and soles | Tyrosine and phenylalanine restriction |
| (B) | 4-hydroxy phenylpyruvate dioxygenase | Transient tyrosinemia of the newborn | Transient immaturity of enzymes usually resolves spontaneously. | Reducing dietary protein to below 2 g/kg/24 h and vitamin C |
| Hawkinsinuria | The abnormal function of enzymes results in metabolic acidosis and failure to thrive in some patients. | A low-protein diet during infancy, long-term use of N-acetyl-cysteine to treat secondary glutathione deficiency, and vitamin C | ||
| Tyrosinemia type III | Primary deficiency of the enzyme, asymptomatic to severe mental retardation and neurologic abnormalities | Tyrosine and phenylalanine restriction and vitamin C | ||
| (C) | Alkaptonuria | Arthritis in older patients and dark urine when exposed to air | Tyrosine and phenylalanine restriction in childhood and adding NTBC or nitisinone in adulthood | |
| (D) | Maleylacetoacetate isomerase | Reported in two siblings with liver failure and renal disease | ||
| (E) | Fumarylacetoacetate hydroxylase | Tyrosinemia type I | Liver, renal, and neurologic diseases | NTBC or nitisinone with a dose of 1 - 2 mg/kg |
2.3. Treatment
(1) Tyrosinemia type I (NTBC or nitisinone with a dose of 1 - 2 mg/kg) (23)
(2) Tyrosinemia type II (tyrosine and phenylalanine restriction) (24)
(3) Tyrosinemia type III (tyrosine and phenylalanine restriction and vitamin C) (26)
(4) Hawkinsinuria (tyrosine and phenylalanine restriction, high dose of vitamin C with a dose of 1000 mg/d, and treatment with N-acetyl-L-cysteine [NAC] with a dose of 150 mg/kg/d) (27)
(5) Alkaptonuria (tyrosine and phenylalanine restriction in childhood and adding NTBC in adulthood) (28)
3. Clinical and Laboratory Diagnoses of Methionine and Homocysteine Cysteine
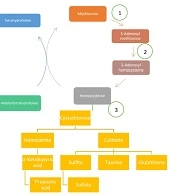
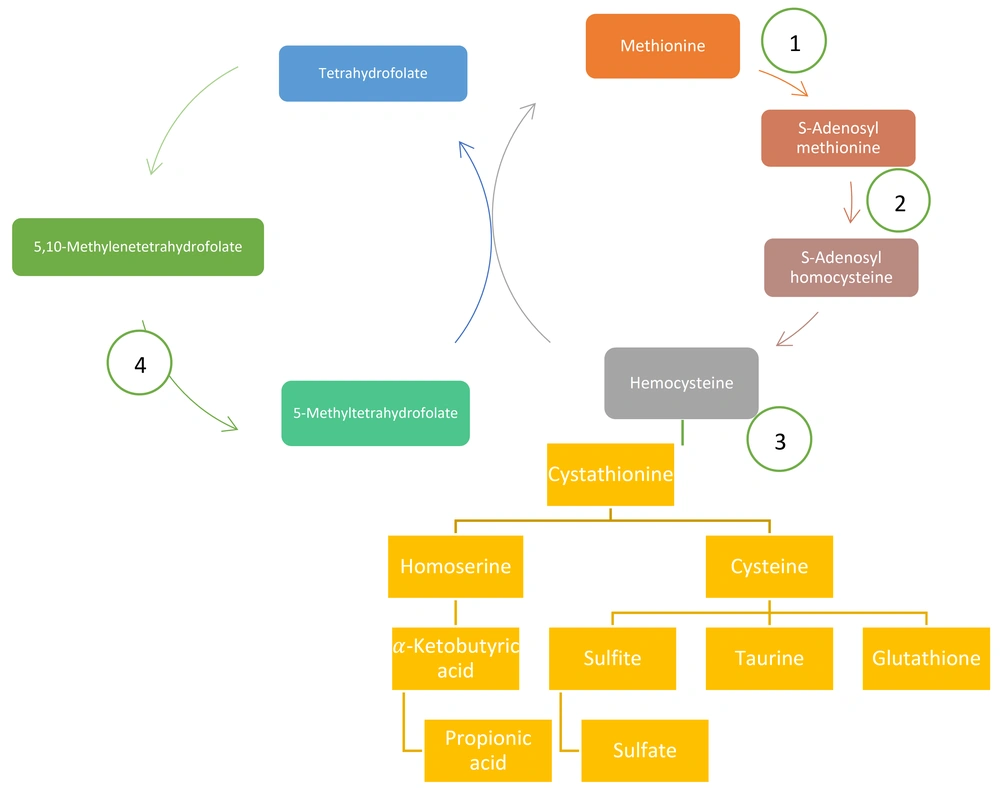
Methionine is more a methyl-donor amino acid than an energy producer. Methionine must lose one methyl and be converted to homocysteine. For this goal, first, it should be attached to adenosyl. Then, methyl is cleaved and attached to glycine and also converted to S-adenosyl homocysteine and finally converted to homocysteine (29).
Homocysteine is dangerous for the body; it mixes with serine and turns into a station, which can convert to cysteine and homo-serine, which has prevented the dangerous effect of homocysteine. Homoserine then enters the propionic cycle and eventually enters the Krebs cycle to produce energy. On the other hand, cysteine converts to oxidant and caretaker metabolites, such as glutathione, taurine, and finally, sulfite and sulfate. The molybdenum cofactor can help convert sulfite to sulfate.
Homocysteine, conversely, can take a methyl group of methyltetrahydrofolate under the influence of vitamin B12 and convert it to methionine. Figure 3 depicts a summary of this pathophysiology.
Hypermethionine has two forms acquired (non-genetic) and genetic, and the genetic form includes hepatic methionine adenosyltransferase (MAT), glycine N-methyltransferase deficiency, S-adenosylhomocysteine, tyrosinemia type I, and citrin deficiency. In addition, the non-genetic form of hypermethioninemia (acquired) occurs in term and preterm infants receiving a high protein diet due to the immaturity of enzyme MAT (30) (Table 2).
| Clinical Presentations | Laboratory Test | Treatment | |
|---|---|---|---|
| Methionine adenosyltransferase (MAT) deficiency (Mudd’s disease) | Most patients have asymptomatic signs and malodor in breathing but, in the severe form, rarely have neurological abnormalities due to demyelination. | Markedly elevated plasma methionine, normal or low level of S-adenosylmethionine, and normal S-adenosylhomocysteine and homocysteine | Combination of a low methionine diet and S-adenosylmethionine |
| Glycine N-methyltransferase deficiency | Most of the other patients have asymptomatic except for mild hepatomegaly and elevated serum level of transaminase. | Hypermethioninemia and very high levels of serum S-adenosylmethionine | No specific treatment has yet been recognized. |
| S-adenosylhomocysteine hydrolase deficiency | Intellectual disability, severe hypotonia, and progressive liver dysfunction | Elevated creatine kinase, hypoalbuminemia (fetal hydrops), and hypoproteinemia | A low-methionine diet has been used but is not effective in the long term. |
| Cystathionine beta-synthase (CBS) deficiency | Up to the age of 3 years, it is a non-specific symptom that causes failure to thrive (FTT) and developmental delay and then has other signs, including lens dislocation and related ocular signs, marfanoid type, thromboembolic complication, and severe mental retardation. | Elevated levels of homocysteine, methionine, or homocysteine in the plasma or urine that can be confirmed by genetic tests | Therapy with pyridoxine (vitamin B6 100 - 150 mg per 24 h), restriction of protein and methionine, betaine 200 - 250 mg/kg/day until maximum 6 g/d in adults, and in B6 unresponsive cases supplementation with folate with dose 15 mg daily or cobalamin (vitamin B12) should be added. |
| Methylcobalamin formation deficiency | Nonspecific presentations include hypotonia, lethargy, and seizure in the first months despite reporting hemolytic uremic syndrome (HUS) | Elevated homocysteine in blood and urine and decreased methionine suggest a combination of homocystinuria and methylmalonic acidemia. As a result, serum methylmalonic should be measured. A genetic test can confirm this diagnosis. | Vitamin B12 in the form of high-dose hydroxocobalamin |
| Methylenetetrahydrofolate reductase (MTHFR) deficiency | Nonspecific presentations include apnea, seizures, coma, ataxia, and thromboembolic attack. | Latest increased blood and urine homocysteine and decreased methionine. Genetic tests can confirm this diagnosis. | B6, B12, methionine, and betaine |
3.1. Cysteine and Cystine
Methionine produces a sulfur-containing amino acid (cysteine). It can be oxidized to a poorly soluble dimer (cystine) that should be transported from the cell by cystinosin. A defect in the gene for the protein cystinosin can be produced in cystinosis, as the cysteine and cystine metabolism are the most known genetic disorders.
The cystine accumulation can cause the formation of crystals involving some organs, such as the brain, eyes, heart, kidney, thyroid, muscles, white blood cells, and pancreas. However, serious problems occur in the kidneys. Cystinosis can occur at any age; nevertheless, about 95% of patients with cystinosis present with the infantile/early-onset form that is known as nephropathic cystinosis due to serious problems in the kidney, such as Fanconi syndrome and renal failure.
3.2. Cystinosis Diagnosis
Cystinosis is diagnosed based on history, physical exam, and laboratory tests, such as the presentation of cystine crystals in the eye and abnormal urine. The evaluation of the amount of cystine in leukocytes is one of the most important tests for detecting cystinosis and can be confirmed by genetic tests.
3.3. Treatment of Cystinosis
There are cystine-depleting medicines, such as cysteamine (brand name Cystagon), that can reduce the amount of cystine inside the cells and lysosomes. There are Cystimin eye drops for removing cystine crystals from the cornea. Acidosis and calcium-phosphorus imbalance was treated by sodium and potassium citrate, calcitriol, and Phosphate Sandoz, respectively. Additionally, a kidney transplant was recommended for renal failure (31).
4. Clinical and Laboratory Diagnoses of Sulfite Oxidase Deficiency and Molybdenum Cofactor Deficiency
As the final step of cysteine metabolism, the oxidation of sulfite to sulfate is performed by sulfite oxidase with a cofactor called molybdenum. The function of two enzymes, including xanthine dehydrogenase and aldehyde oxidase, also depends on this cofactor. Most patients with sulfite oxidase deficiency also have molybdenum cofactor deficiency. Clinical presentations in these patients in the neonatal period include hypoxic-ischemic encephalopathy symptoms, including severe developmental delay, severe intractable tonic, clonic, myoclonic seizures, cortical atrophy with subcortical multiple cystic lesions, and bilateral dislocation of ocular lenses.
4.1. Diagnosis
Sulfite, xanthine, thiosulfate, hypoxanthine, and S-sulfocysteine levels are increased, and urinary uric acid and sulfate levels are decreased in affected children. Fresh urine must be used as sulfite can oxidize to sulfate at room temperature, giving false-negative results. Diagnosis can be confirmed by measuring the levels of sulfite oxidase and molybdenum cofactor in fibroblasts or liver biopsies or by DNA studies.
4.2. Treatment
There is no effective treatment. However, high doses of vitamin B6 (5 - 100 mg/kg) for preventing seizures, not neurologic outcome, was recommended. Recently, treatment by cyclic pyranopterin monophosphate (cPMP) was given as a multicenter clinical trial; nevertheless, the effect is unknown.
4.3. Tryptophan
Tryptophan, an essential amino acid, is a precursor for niacin (nicotinic acid) and serotonin.
5. Clinical and Laboratory Diagnoses of Tryptophan
Hartnup disorder, as an autosomal recessive disease, is due to the problem in the transport of neutral amino acids, such as tryptophan, in renal tubules and intestinal mucosa. The presentations of Hartnup disorder varied from asymptomatic to cutaneous photosensitivity, pellagra-like rash after moderate exposure to sunlight, intermittent ataxia, psychiatric manifestations, short stature, and atrophic glossitis due to tryptophane deficiency.
5.1. Diagnosis
The diagnosis of Hartnup disease is based on clinical manifestations, such as the intermittent nature of symptoms. The main laboratory finding in urine amino acid chromatography is monoamino-monocarboxylic aminoaciduria (e.g., alanine, histidine, threonine, serine, isoleucine, tyrosine, leucine, valine, phenylalanine, and tryptophan). However, the urinary excretion of proline, arginine, and hydroxyproline stays normal. Hartnup disease can be differentiated from Fanconi syndrome by this finding. Reflected compensatory mechanisms result in normal or slightly decreased plasma neutral amino acid concentrations. In some patients, the bacterial breakdown of unabsorbed tryptophan causes large amounts of indole derivatives in the intestines. Genetic analysis can confirm the diagnosis of Hartnup disease.
5.2. Treatment
A high-protein diet and nicotinic acid or nicotinamide (50 - 300 mg/24 h) were recommended in symptomatic patients with Hartnup disorder (32).
6. Clinical and Laboratory Diagnoses of Glycine
Glycine is a nonessential amino acid in the body that is mostly made from serine and threonine. Glycine is the lowest molecular weight proteinogenic amino acid that, in addition to participating in the construction of proteins, is needed for various metabolic pathways, including glutathione synthesis and regulation of one-carbon metabolism (33). It also acts as a neurotransmitter in the nervous system.
The body might develop hypoglycinemia or hyperglycinemia. Primary glycine deficiency has not been reported in isolation. Hypoglycemia is due to a defect in the serine biosynthesis pathway which leads to the absence of serine in body fluids, such as cerebrospinal fluid (CSF), and glycine deficiency. Elevated glycine levels in body fluids cause hyperglycemia. Its pathophysiology is not fully understood. However, in these patients, a genetic defect in the cleavage enzyme system or the inhibition of the cleavage enzyme system occurs. Depending on the pathophysiology which occurs, they are divided into two categories, namely NKH and ketotic hyperglycinemia. Non-ketotic hyperglycemia occurs by a genetic defect of the glycine cleavage enzyme system. Ketotic hyperglycinemia occurs by propionic acidemia, methylmalonic acidemia, isovaleric acidemia, and 4) beta-ketothiolase deficiency (34).
Non-ketotic hyperglycinemia, sometimes called glycine encephalopathy, has four forms, namely neonatal, infantile, late-onset, and transient. Neonatal NKH, as the most frequent form, presents in the first few days of life (within 6 hours and 8 days after birth). In this form, hiccups and convulsions, especially myoclonic seizures, are common. Other symptoms include poor feeding, lack of sucking, lethargy, apnea, and death.
Moderate to severe hyperglycemia is diagnosed by laboratory findings. An increase in glycine concentration of 15-30 times normal and a ratio of CSF to plasma glycine concentration greater than 0.08 indicate NKH. About 30% of infants with NKH expire with supportive care, and the infants who remain alive suffer from uncontrollable seizure disorders and psychomotor retardation.
In the infantile form of NKH, at first, the infant is normal; however, after 6 months, the signs and symptoms appear, and seizures and hypotension are common manifestations. The infantile form of NKH is milder than the neonatal form, and infants usually survive. In addition, mental retardation is not more severe than the neonatal form. Late onset is the atypical type of NKH, which occurs between the ages of 2 and 33 years, and its clinical manifestations include optic nerve atrophy, choreoathetotic movements, and progressive spastic diplegia.
Transient and neonatal forms of NKH are indistinguishable from laboratory and clinical manifestations. However, in the transient form, after the cessation of the glycine-lowering drug at the age of 2 - 8 weeks, the levels of plasma and CSF glycine normalize, and the clinical signs and symptoms improve.
The glycine concentrations in blood and urine can be moderately increased by sodium valproate. For diagnosis, the repeated measurement of glycine levels after discontinuation of the drug is helpful. Sodium valproate, pyridoxamine-5′-phosphate oxidase (PNPO) deficiency, and transient glycine encephalopathy should be ruled out for the diagnosis of all forms of NKH. In mild forms of the disease, some drugs, such as dextromethorphan and felbamate, which reduce the effect of glycine on nerve cells, are helpful. However, no effective treatment is known for severe forms.
7. Clinical and Laboratory Diagnoses of Hyperoxaluria
7.1. Pathophysiology
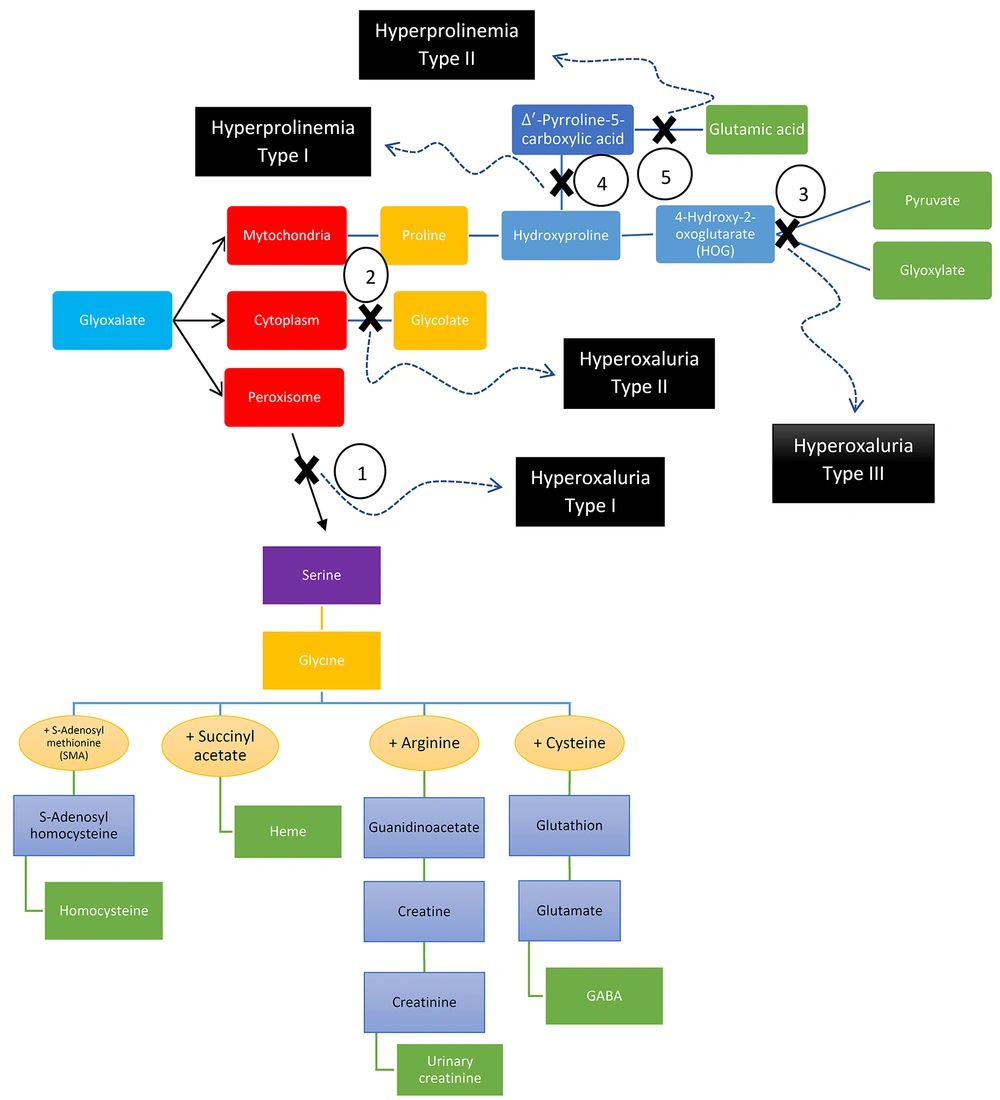
The oxalate in vegetables is absorbed directly through the intestine. It then enters the cytoplasm as glycolate and is converted to glyoxylate in the peroxisomes. Finally, glyoxylate is converted to glycine. Type 1 hyperoxaluria occurs in the absence of the enzyme that performs this conversion. In this case, glyoxylate is converted to glycolate in the cytoplasm. It accumulates due to the limited capacity of the enzyme that converts glyoxylate to glycolate, resulting in hyperoxaluria. If the enzyme that converts glyoxylate to glycolate is not genetically present, type 2 hyperoxaluria develops.
On the other hand, collagen in food is converted to proline and then enters the mitochondria to glyoxylate. It then transforms into two substances (i.e., glycine and glycolate). Mitochondria have enzymes in both the peroxisome and cytoplasm; one enzyme in the peroxisome converts glyoxylate to glycine, and another in the cytoplasm converts glyoxylate to glycolate. Despite the lack of glyoxylate formation in mitochondrial due to an unknown cause, hyperoxaluria type 3 is formed due to precursor accumulation in mitochondria that prevents the conversion of glyoxylate to glycine in the peroxisome.
The external sources of oxalic and glycolic acids include vegetables and foods containing oxalate, such as almond milk and spinach. However, most glycolic and oxalic acids are produced endogenously. Two pathways protect the body against the overproduction of oxalic acid. Generally, most glyoxylate is transported to peroxisomes and changes to glycine by alanine-glyoxylate transaminase. Therefore, if this enzyme is defective, glyoxylate increases and causes type 1 hyperoxaluria. The enzyme glyoxylate reductase/hydroxy pyruvate reductase converts the major remaining part of the glyoxylate in the cytosol to glycolate. Therefore, a defect in this enzyme causes type 2 hyperoxaluria. The mitochondrial enzyme 4-hydroxy-2-oxoglutarate aldolase 1 (HOGA1) is involved in the last stage of the hydroxyproline metabolic pathway, which produces pyruvate and glycolate, and the produced pyruvate enters the Krebs cycle. Type 3 hyperoxaluria is caused by a defect in this mitochondrial enzyme (Figure 4).
The first clinical manifestations of hyperoxaluria are nephrocalcinosis, kidney stones, and renal colic. The major laboratory finding in the diagnosis of hyperoxaluria is the elevated excretion of oxalate (normal excretion: 10 - 50 mg/24 h) in urine. In addition, in type 2, urinary L-glyceric acid is elevated, and glycolic acid and glyoxylic acid levels are normal. In type 3, serum and urine 4-hydroxy-2-oxoglutarate (HOG) levels increase.
The basis of treatment is to decrease the production of oxalic acid and increase the excretion of calcium oxalate. High doses of pyridoxine are prescribed to reduce plasma levels and increase the urinary excretion of oxalate. The alkalinization of urine, excessive consumption of oral fluids (2 - 3 liters per square meter in 24 hours with control of fluid balance), phosphate supplementation, avoiding the drugs that elevate calcium excretion in urine (e.g., loop diuretics), and monitoring of the intake of vitamin C and D are recommended to increase the excretion of calcium oxalate and prevent nephrolithiasis.
8. Clinical and Laboratory Diagnoses of Creatine Deficiency Disorders
Creatine is synthesized from arginine and glycine, mostly in the kidneys, liver, pancreas, and brain. It is then transported to the brain and muscles for phosphorylation and dephosphorylation by creatine kinase and the production of creatine phosphokinase for transport phosphor and production of energy after this work.
Creatine can be metabolized to creatinine and excreted in urine. There are three commonly known genetic disorders that cause creatine deficiency. First, guanidinoacetate was produced by arginine: glycine amidinotransferase (AGAT), and then guanidinoacetate can be methylated by guanidinoacetate methyltransferase (GAMT) and produce creatine. In the last step, creatinine can be transported to the brain and muscle by creatine transporter (CRTR).
8.1. Clinical Manifestations
Complications are related to the brain and muscle, such as intellectual, developmental, and speech delay, psychiatric symptoms (e.g., autism and psychosis), ataxia, hypotonia, and dystonic movement, especially in GAMT and CRTR deficiencies. In addition, seizures are common findings.
8.2. Diagnosis
Laboratory findings include decreased creatine in plasma and decreased urinary creatine to creatinine ratio in AGAT and GAMT deficiencies. The creatinine level in plasma is insufficient for the diagnosis of these disorders. The significant increase and decrease of guanidinoacetate in the blood, urine, and especially in CSF, are diagnostic of GAMT and AGAT deficiencies, respectively. The creatine-to-creatinine ratio in urine is increased in male cases who have CRTR deficiency, although it can also be slightly increased in female carriers. In the brain, magnetic resonance spectroscopy (MRS) can show a lack of creatine and creatine phosphate (in all three defects) and increased levels of guanidinoacetate in GAMT deficiency. In the globus pallidus, brain magnetic resonance imaging (MRI) might also show signal hyperintensity. Diagnosis can be confirmed by DNA analysis.
8.3. Treatment
Treatments are age-dependent. The best result is obtained when the treatment is initiated pre-symptomatically or in the neonatal period. Only oral creatine monohydrate (up to 400 - 800 mg/kg/24 h) might lessen muscle weakness and improve neurocognitive outcomes in AGAT-deficient patients. In GAMT-deficient patients, neurocognitive development, muscle tone, and seizures can be improved by dietary arginine restriction and oral creatine monohydrate (up to 400 - 800 mg/kg/24 h) plus ornithine (up to 400 - 800 mg/kg/24 h). There is no treatment in CRTR-deficient patients; however, some patients might have improvements in seizures and neurocognitive outcomes. Arginine: glycine amidinotransferase and GAMT defects are inherited as autosomal recessive traits. Nevertheless, CRTR deficiency is X-linked.
9. Clinical and Laboratory Diagnoses of Serine
Serine is a nonessential amino acid. It is obtained through endogenous sources and dietary intake. Serine is synthesized from glucose and glycine endogenously. The endogenous production of serine is principal for synaptic amino acid and the metabolism of phospholipids. Serine deficiency disorders have a wide clinical spectrum. There is Neu-Laxova syndrome as the severe end and developmental delay and epilepsy as the milder end of the spectrum.
9.1. Laboratory Tests
Blood and CSF deficiencies were observed in these patients, and DNA analysis can confirm these problems. Treatment by serine (200 - 700 mg/kg/24 h orally) and glycine (200 - 300 mg/kg/24 h) started in early life can help.
10. Clinical and Laboratory Diagnoses of Proline
Proline is one of the essential amino acids in humans made endogenously in the body from ornithine, glutamic acid, and arginine. Hydroxyproline and proline are abundant in tissue collagen. Proline also participates in the glutamatergic synapses of the central nervous system as a neurotransmitter and glutamate precursor (35). The normal excretion of proline and hydroxyproline is in the form of amino peptides (i.e., peptides or dipeptides that involve proline and hydroxyproline) and is not found in the form of amino acids in the urine. In some diseases, such as rickets and hyperparathyroidism, the excretion of these aminopeptides is increased. However, if proline accumulates in the tissues, it causes type 1 and type 2 hyperprolinemia.
Cutis laxa is a syndrome caused by a decrease in new proline synthesis whose clinical manifestations are progeroid features or spastic paraplegia. Type 1 and type 2 hyperprolinemia are inherited as autosomal recessive disorders, with type 1 due to defects in the gene encoding proline oxidase (PRODH) and type 2 due to defects in the gene encoding P5C dehydrogenase (ALDH4A1). Their clinical manifestations are similar and might be asymptomatic or associated with behavioral problems, mental retardation, autism spectrum, and seizures (36).
In type 1 hyperprolinemia, increased proline levels are found in plasma, CSF, and urine. Glycine and hydroxyproline are also excreted in the urine. There is still no effective treatment, and dietary proline restriction does not improve clinical manifestations. In type 2 hyperprolinemia, the concentrations of Δ1-pyrroline-5-carboxylic acid (P5C) and proline in the blood, CSF, and urine increase, and the presence of P5C differentiates it from type 1. In body fluids, especially in CFS, high levels of P5C act as antagonists of vitamin B6, causing seizures and neurological manifestations in these patients. It is recommended to use high doses of vitamin B6 for treatment.
11. Clinical and Laboratory Diagnoses of Glutathione
The glutamic acid induction of alpha-ketoglutarate by taking an amine is first converted to glutamic acid, and then by re-taking an amine, it is converted to glutamine. Glutamic acid and glutamine can have numerous biologic roles, such as intermediators in biochemical function, neurotransmitters, and precursors of glutathione as the most important antioxidant in the body by the gamma-glutamyl cycle. Glutathione, as an antioxidant, can protect other sulfhydryl-containing compounds from oxidation and help detoxify peroxides, although glutathione is able to transport amino acids across the cell membrane (37).
The increased excretion of 5-oxoproline in urine is one of the presentations of gamma-glutamyl cycle deficiency (38), which has genetic and non-genetic reasons. 5-Oxoprolinemia is a differential diagnosis of high anion gap metabolic acidosis (HAGMA) and, therefore, must be kept in mind. Massive 5-oxoprolinuria might be due to two metabolic disorders, namely 5-oxoprolinase deficiency and glutathione synthetase deficiency. Nevertheless, there is a more prevalent clinical scenario which is a slight temporary increase of 5-oxoproline in urine that might be in different acquired and metabolic conditions, such as exposure to paracetamol and several hydrolyzed-protein formulas, tyrosinemia type I, Stevens-Johnson syndrome, urea cycle defects, severe burns, and homocystinuria.
There is hemolytic anemia (mild form) with or without metabolic acidosis and developmental delay in different kinds of glutathione synthetase deficiency; nonetheless, increasing 5-oxoproline in urine was observed in most of them. For the treatment of acute attacks, the patients should be hydrated. Acidosis should be corrected by the infusion of sodium bicarbonate. In addition, anemia and hyperbilirubinemia correction are part of the treatment. Stressful catabolic states, drugs, and oxidants that might cause hemolysis should be avoided. Vitamin E, vitamin C, and selenium are prescribed due to their anti-oxidant effect. Oral glutathione analogs have been administered with variable success. There are other rare diseases in the gamma-glutamyl cycle.
12. Clinical and Laboratory Diagnosis of Urea Cycle Defect
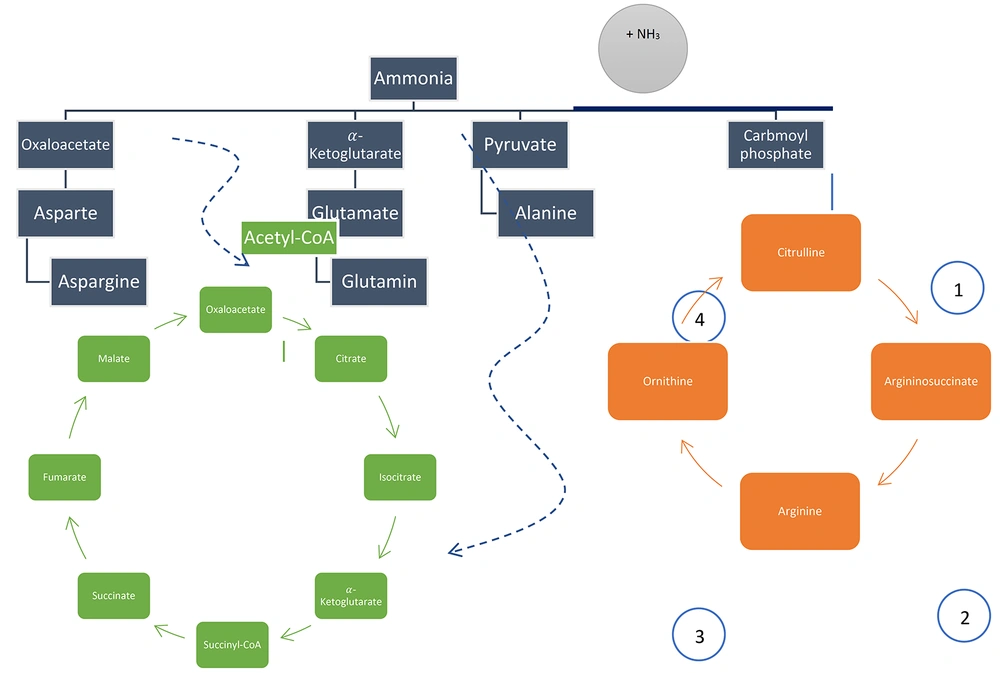
Ammonia is produced by amino acid catabolism. Ammonia is harmful to the human body and should be detoxified by the urea cycle through the production of urea. There are other pathways for the detoxification of ammonia, such as the production of asparagine ([oxaloacetate + ammonia = aspartate] + ammonia), alanine (pyruvate + ammonia), and glutamine ([α-ketoglutarate + ammonia = glutamate] + ammonia), which help in the detoxification of ammonia in the liver and another cell, such as muscle. Figure 5 depicts a summary of the aforementioned items.
The increase of alanine plus other amino acids due to liver failure, such as tyrosine, methionine, and galactose, was observed in citrullinemia type 2. Changes in plasma citrulline, other amino acids, and urine orotic acid are diagnostic (Table 3). Differential diagnosis of hyperammonemia is mentioned in Table 4, similar to what is mentioned in Yoo’s Study (39).
| Diagnosis | Plasma Amino Acid | Urine Amino Acid | Urine Organic Acid |
|---|---|---|---|
| Argininosuccinate lyase (ASL) deficiency | Increasing citrulline/arginosuccinate acid (ASA) | Increasing arginosuccinate acid (ASA) | Increasing orotic acid |
| Arginase deficiency | Increasing arginine | Arginine lysine; Cysteine; Ornithine | Increasing orotic acid |
| HHH syndrome | Increasing ornithine and citrulline | Ornithine Homocitrulline | Increasing orotic acid |
| Argininosuccinate synthetase deficiency (citrullinemia) | |||
| Type 1 | Increasing citrulline, alanine, aspartic acid, and glutamine | Decreasing orotic acid | |
| Type 2 | Increasing citrulline, alanine, alfa fetoprotein, galactose, tyrosine, and methionine | Decreasing orotic acid without succinyl acetone | |
| N-Acetyl glutamate synthase deficiency (NAGS) deficiency (Decreasing citron) | Normal orotic acid | ||
| Carbamoyl phosphate synthetase (CPS) deficiency (Decreasing citrulline) | Normal or decreased orotic acid | ||
| Ornithine transcarbamylase (OTC) deficiency (Decreasing citrulline) | Increasing orotic acid |
| Plasma Citrulline | Other Features | Diagnosis |
|---|---|---|
| Low (usually) | ↑↑ Orotic acid | Ornithine transcarbamylase deficiency |
| Specific acylcarnitines and organic acids | Organic aciduria (e.g., propionic or methylmalonic aciduria) | |
| ↓ - n Orotic acid | Carbamoyl phosphate synthetase deficiency; N-acetylglutamate synthase deficiency; Ornithine aminotransferase deficiency (newborns) | |
| > 30 µM | ↑ Orotic acid | Lysinuric protein intolerance |
| > 50 µM | ↓ - n Orotic acid, ↑ lactate | Pyruvate carboxylase deficiency (neonatal) |
| 100 - 300 µM | ↑ Argininosuccinate | Argininosuccinic acidemia |
| > 1000 µM | ↑ Orotic acid | Citrullinemia |
12.1. Treatment of Hyperammonemia
Table 5 shows a summary of the treatment of urea cycle defect.
| Disorder | Sodium Benzoate (to Be Given IV in Glucose 10%) | Sodium PBA/Sodium Phenylacetate (to Be Given IV in Glucose 10%) | L-arginine Hydrochloride (to Be Given IV in Glucose 10%) | N-carbamyl Glutamate (Only Available as an Oral/Enteral Drug) |
|---|---|---|---|---|
| Undiagnosed patients A | 250 mg/kg as bolus in 90 - 120 min, then maintenance: 250 - 500 mg/kg/d B > 20 kg bw: 5.5 g/m2/d | 250 mg/kg as bolus in 90 - 120 min, then maintenance: 250 - 500 mg/kg/d B | 250 - 400 mg/kg (1 - 2 mmol/kg) as bolus in 90 - 120 min, then maintenance: 250 mg/kg/d (1.2 mmol/kg/d) | 100 mg/kg bolus per nasogastric tube, then 25 - 62.5 mg/kg every 6 h |
| NAGSD (N-Acetyl glutamate synthase deficiency) | Same B | Same B | 250 mg/kg (1.2 mmol/kg as bolus in 90 - 120 min, then maintenance: 250 mg/kg/d (1.2 mmol/kg/d) | Same |
| CPSID and OTCD | Same B | Same B | Same | - |
| ASSD | Same B | Same B | Same | - |
| ASLD C | Same B | Same B | 200 - 400 mg/kg (1 - 2 mmol/kg) as bolus in 90 - 120 min, then maintenance: 200 - 400 mg/kg/d (1 - 2 mmol/kg/d) | - |
| ARG1D D | Same B | - | AVOID | - |
| HHH syndrome | Same B | Same B | 250 mg/kg (1.2 mmol/kg as bolus in 90 - 120 min, then maintenance: 250 mg/kg/d (1.2 mmol/kg/d) | - |
Abbreviation: IV, intravenously.
aThe doses indicated in this table can be used at the start of treatment but must be adapted depending on plasma ammonia and amino acids. Sodium benzoate and sodium PBA/phenylacetate should be given in parallel in severe acute decompensation. In less severe cases, a step-wise approach with initial sodium benzoate and, if hyperammonemia persists or worsens, the addition of sodium PBA/phenylacetate can be chosen. Since hyperammonemia causes brain edema, sufficient NaCl should be added so that solutions are not hypotonic. The sodium load in the other intravenous medications should be taken into account. Maximal daily drug dosages: sodium benzoate 12 g/d, sodium PBA 12 g/d, L-arginine 12 g/d. A, In undiagnosed patients, the use of a combination of the drugs in this table seems justified; consider the additional use of carnitine 100 mg/kg IV/d, hydroxocobalamin 1 mg IM/IV/d, and biotin 10 mg IV/PO/d. B, If on hemodialysis/hemodiafiltration, doses should be increased to 350 mg/kg/d (maintenance dose). C, In ASLD, l-arginine therapy for acute decompensations might be sufficient for some patients. D, The risk for acute hyperammonemia decompensation is low in ARG1D.