1. Background
Multiple sclerosis (MS) is an inflammatory immune-mediated demyelinating and debilitating neurodegenerative disease of the central nervous system (CNS) (1-3) characterized by a wide range of neurological symptoms, from sensorimotor complications to cognitive dysfunction (4). This disease is categorized into four phenotypes: Clinically isolated syndrome (CIS), primary progressive multiple sclerosis (PPMS), secondary progressive multiple sclerosis (SPMS), and relapsing-remitting multiple sclerosis (RRMS) (5, 6).
The clinically isolated syndrome is an MS phenotype defined by the clinical symptoms suggestive of the first episode of MS. Some individuals diagnosed with CIS are at high risk of progression to MS. It has been estimated that up to 85% of patients with MS have experienced CIS as the first episode of their disease, and approximately 70% of those with CIS will develop a second evident demyelinating course and eventually move toward MS (7-10).
The radiologically isolated syndrome (RIS) is defined as the incidental finding of lesions fulfilling the criteria for MS by magnetic resonance imaging (MRI) in the absence of clinical manifestations. This syndrome is usually an incidental finding in MRI performed for other reasons unrelated to MS symptoms, such as migraine, other types of headaches, or trauma. This syndrome is a rare condition with a rate of 0.8 cases per 100000 person-years (11, 12) and is believed to be a precursor of RRMS or PPMS; however, not all patients diagnosed with RIS would develop MS (13). The RIS introduced by Okuda et al. in 2009 (14, 15), followed by considerable changes in its diagnostic criteria by McDonald et al. in 2017, noting that dissemination in space (DIS) can also be a manifestation of RIS (5). It has been estimated that one-third of people diagnosed with RIS will progress to CIS, RRMS, or PPMS; one-third will retain radiological but not clinical manifestations of MS, and the remaining one-third will remain stable both radiologically and clinically (16).
Neurologists have diverse ideas about the therapeutic approach for CIS and RIS. The early initiation of disease-modifying therapies (DMTs) is favored by some neurologists who believe that waiting for MS manifestations to appear may cause irreversible and irrecoverable CNS complications. Others recommend close monitoring of the patients because DMTs may lead to significant adverse effects (11, 17-19). Considering the notions given above and the fact that a few studies have addressed these controversial issues, it is beyond important to investigate the demographic, clinical, and radiological features of individuals diagnosed with MS, RIS, or CIS.
2. Objectives
In the current large population-based study, we aimed to compare the clinical and demographic features of patients diagnosed with MS, RIS, and CIS.
3. Methods
This was a retrospective census-based cross-sectional study conducted on 3716 patients with the diagnoses of MS, CIS (5), or RIS (14) referred to the MS Clinic of Kashani Hospital affiliated with Isfahan University of Medical Sciences (IUMS) from June 2018 to April 2019. All patients diagnosed with MS, CIS, or RIS who gave informed written consent were included.
The Ethics Committee of Isfahan University of Medical Sciences approved the study protocol. The patients were informed about the study protocol and reassured about the confidentiality of their personal information. The written Helsinki (1989) consent form of participation was obtained from all the participants.
Exclusion criteria were the diagnosis of chronic demyelinating neurologic disorders other than MS, CIS, or RIS, as well as incomplete medical records (e.g., the year of disease onset, MRI findings, family history, etc.).
The diagnosis of RIS was based on Okuda and colleagues’ criteria (14), while DIS was defined in the current study based on that noted by McDonald et al. in 2018 (5).
The patient's demographic information, including age, gender, occupation, and marital status, as well as clinical data (the age of onset, MRI findings, medical history, family history, smoking, and body mass index (BMI)), were recorded into a checklist.
The MRI images were evaluated by an expert neuroradiologist, as well as a skilled neurologist, to prepare a thorough report and determine deterministic manifestations, including the location and activity of the plaques, lesion load, the presence of atrophy, number of cervical and thoracolumbar plaques, longitudinally extensive transverse myelitis (LETM), and the transverse plane of cervical plaques.
Longitudinally extensive transverse myelitis was defined as hyperintense spinal cord lesions extending toward three or more vertebral levels in the sagittal T2-Weighted MRI view (20). Brain atrophy was measured manually by an experienced neurologist who was familiar with brain anatomy and evaluated the appearance of the CNS tissue in all brain MRIs. Brain atrophy was indicated when there was an increase in the cerebrospinal fluid (CSF) space or a reduction in the parenchymal structure. According to the extent of ventricular size increase or parenchymal volume reduction, brain atrophy was rated using a Likert scale as grade 1 (mild; < 10%), grade 2 (moderate; 10 - 25%), or grade 3 (severe; > 25%). Lesion load was measured on T2-FLAIR images using the lesion segmentation toolbox (LST) of the statistical parametric mapping (SPM) software for automatic lesion detection and then was manually corrected. The patients were subdivided into three groups according to the total lesion volume: Low (< 5 mL), medium (between 5 and 15 mL), and high (> 15 mL) lesion load.
3.1. Statistical Analysis
Statistical analyses were carried out using SPSS Software Version 18 (Armonk, NY: IBM Corp). The characteristics of the participants were assessed using frequency (percent) for categorical variables and mean ± standard deviation for continuous variables. Continuous variables were compared between different groups of patients using ANOVA (or Kruskal-Wallis test in the absence of normal data distribution). Categorical data were analyzed using the Chi-square test (or Fisher’s exact test when appropriate). For post-hoc analysis, Bonferroni correction was applied to adjust for pairwise comparisons. Observations with a two-tailed P-value of < 0.05 were considered to be statistically significant.
4. Results
In the current study, a total of 3716 patients, including 404 (10.87%) cases with RIS, 74 (1.99%) cases with CIS, and 3238 (87.13%) individuals with MS, were included. Patients with MS were divided into three phenotypic subgroups: Remitting-relapsing multiple sclerosis (RRMS), primary-progressive multiple sclerosis (PPMS), and secondary-progressive multiple sclerosis (SPMS), comprising 2584 (69.53%), 122 (3.28%) and 532 (14.31%) patients, respectively.
Comparing the study groups revealed a remarkably lower age of onset (P-value < 0.001) among those presenting with RRMS, while BMI was significantly higher in patients with RIS (P-value < 0.001). No significant differences were noticed in terms of gender distribution, smoking status, and family history of MS between the study groups. Detailed information on the patients has been presented in Table 1.
| Variables | RRMS (n = 2584) | SPMS (n = 532) | PPMS (n = 122) | CIS (n-404) | RIS (n = 74) | P-Value |
|---|---|---|---|---|---|---|
| Age, y | 38.25 ± 9.07 | 46.91 ± 9.96 * | 48.34 ± 9.81 | 35.74 ± 8.97 | 36.14 ± 8.80 | < 0.001 |
| Body mass index, kg/m2 | 24.40 ± 4.17 * | 23.91 ± 4.35 * | 24.53 ± 3.49 | 24.94 ± 4.20 | 25.17 ± 3.96 | 0.003 |
| Age of onset, y | 29.69 ± 8.60 * | 31.65 ± 9.62 | 40.14 ± 11.08 | 30.44 ± 8.56 | 31.63 ± 8.96 | < 0.001 |
| Gender | < 0.001 | |||||
| Male | 525 (20.3) | 131 (24.6) | 58 (47.5) | 88 (21.8) | 15 (20.3) | |
| Female | 2059 (79.7) | 401 (75.4) | 64 (52.5) | 316 (78.2) | 59 (79.7) | |
| Marital status | < 0.001 | |||||
| Single | 632 (24.6) | 89 (16.9) | 13 (10.7) | 129 (32) | 24 (34.3) | |
| Married | 1866 (72.6) | 405 (77) | 103 (84.4) | 263 (65.3) | 44 (62.9) | |
| Divorced/widow | 71 (2.8) | 32 (6.1) | 6 (4.9) | 11 (2.7) | 2 (2.8) | |
| Occupation | < 0.001 | |||||
| Employed | 2383 (92.6) | 452 (85.3) | 105 (86.1) | 376 (94.5) | 66 (90.4) | |
| Unemployed | 190 (7.4) | 78 (14.7) | 17 (13.9) | 22 (5.5) | 7 (9.6) | |
| Smoking | 0.002 | |||||
| Yes | 222 (8.9) | 41 (8.1) | 22 (18.6) | 36 (9.3) | 2 (2.8) | |
| No | 2261 (91.1) | 466 (91.9) | 96 (81.4) | 351 (90.7) | 70 (97.2) | |
| Family history of multiple sclerosis | 0.083 | |||||
| Positive | 629 (25.1) | 133 (25.9) | 21 (17.4) | 78 (20.5) | 15 (20.5) | |
| Negative | 1877 (74.9) | 380 (74.1) | 100 (82.6) | 302 (79.5) | 58 (79.5) |
Abbreviations: RRMS, remitting-relapsing multiple sclerosis; SPMS, secondary-progressive multiple sclerosis; PPMS, primary-progressive multiple sclerosis; CIS, clinically isolated syndrome; RIS, radiologically isolated syndrome.
a Values are expressed as mean ± SD or No. (%).
b Continuous variables were compared between different groups of patients using ANOVA. Categorical data were analyzed using the chi-square test.
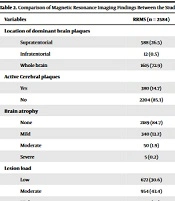
Imaging findings in the patients have been shown in Table 2. Supratentorial lesions were more frequent in RRMS, followed by CIS, while brain lesions were more predominantly observed in the RRMS group, followed by SPMS (P-value < 0.001). In addition, the ratio of patients with active brain plaques was considerably higher among those with RRMS compared to other groups (P-value = 0.01). The highest proportions of patients with brain atrophy (P-value < 0.001), moderate-to-high lesion load (P-value < 0.001), multiplicity in the cervical plaques (P-value < 0.001), and LETM (P-value < 0.001) were observed in the SPMS group, followed by PPMS.
| Variables | RRMS (n = 2584) | SPMS (n = 532) | PPMS (n = 122) | CIS (n = 404) | RIS (n = 74) | P-Value b |
|---|---|---|---|---|---|---|
| Location of dominant brain plaques | < 0.001 | |||||
| Supratentorial | 588 (26.5) | 33 (7.3) | 19 (17.6) | 116 (40.7) | 32 (50) | |
| Infratentorial | 12 (0.5) | 0 (0) | 0 (0) | 15 (5.3) | 1 (1.6) | |
| Whole brain | 1615 (72.9) | 421 (92.7) | 89 (82.4) | 154 (54) | 31 (48.4) | |
| Active Cerebral plaques | < 0.001 | |||||
| Yes | 380 (14.7) | 33 (6.2) | 5 (4.1) | 38 (9.4) | 3 (4) | |
| No | 2204 (85.3) | 499 (93.8) | 117 (95.9) | 366 (90.6) | 71 (96) | |
| Brain atrophy | < 0.001 | |||||
| None | 2189 (84.7) | 151 (28.4) | 53 (43.4) | 391 (96.8) | 72 (97.3) | |
| Mild | 340 (13.2) | 197 (37) | 43 (35.2) | 13 (3.2) | 1 (1.4) | |
| Moderate | 50 (1.9) | 145 (27.3) | 24 (19.7) | 0 (0) | 0 (0) | |
| Severe | 5 (0.2) | 39 (7.3) | 2 (1.6) | 0 (0) | 1 (1.4) | |
| Lesion load | < 0.001 | |||||
| Low | 672 (30.6) | 28 (6.2) | 25 (23.1) | 169 (60.1) | 44 (68.8) | |
| Moderate | 954 (43.4) | 86 (19.1) | 29 (26.9) | 89 (31.7) | 16 (25) | |
| High | 570 (26) | 336 (74.7) | 54 (50) | 23 (8.2) | 4 (6.3) | |
| Number of cervical plaques | < 0.001 | |||||
| 0 | 1135 (43.9) | 115 (21.6) | 22 (18) | 306 (75.7) | 59 (79.7) | |
| 1 | 533 (20.6) | 28 (5.3) | 13 (10.7) | 56 (13.9) | 11 (14.9) | |
| 1 - 3 | 562 (21.7) | 85 (16) | 25 (20.5) | 28 (6.9) | 2 (2.7) | |
| > 3 | 354 (13.7) | 304 (57.1) | 62 (50.8) | 14 (3.5) | 2 (2.7) | |
| Longitudinally extended transverse myelitis | < 0.001 | |||||
| Positive | 172 (6.7) | 138 (25.9) | 30 (24.6) | 16 (4) | 4 (5.4) | |
| Negative | 2412 (93.3) | 394 (74.1) | 92 (75.4) | 388 (96) | 70 (94.6) | |
| Active cervical plaque | 0.005 | |||||
| Yes | 132 (5.1) | 16 (3) | 4 (3.3) | 6 (1.5) | 2 (2.7) | |
| No | 2452 (94.9) | 516 (97) | 118 (96.7) | 398 (98.5) | 72 (97.3) | |
| Transverse plane of cervical plaque | < 0.001 | |||||
| Peripheral | 1182 (83.1) | 117 (28.4) | 33 (33.7) | 90 (89.1) | 13 (92.9) | |
| Central | 3 (0.2) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |
| Central and peripheral | 237 (16.7) | 295 (71.6) | 65 (66.3) | 11 (10.9) | 1 (7.1) |
a Values are expressed as No. (%).
b Chi-square test
Comorbidities and family history of diseases were compared between the studied groups, showing that diabetes, hypertension, and family history of autoimmune disorders other than MS were significantly higher among PPMS patients than in other groups (Table 3).
| Variables | RRMS (n = 2584) | SPMS (n = 532) | PPMS (n = 122) | CIS (n = 404) | RIS (n = 74) | P-Value b |
|---|---|---|---|---|---|---|
| Diabetes | 0.006 | |||||
| Yes | 62 (2.4) | 25 (4.7) | 7 (5.7) | 7 (1.7) | 2 (2.7) | |
| No | 2522 (97.6) | 507 (95.3) | 115 (94.3) | 397 (98.3) | 72 (97.3) | |
| Hypertension | 0.027 | |||||
| Yes | 118 (4.6) | 30 (5.6) | 10 (8.2) | 12 (3) | 0 (0) | |
| No | 2466 (95.4) | 502 (94.4) | 112 (91.8) | 392 (97) | 74 (100) | |
| Anemia | 0.055 | |||||
| Yes | 230 (8.9) | 38 (7.1) | 4 (3.3) | 43 (10.6) | 4 (5.4) | |
| No | 2354 (91.1) | 494 (92.9) | 118 (96.7) | 361 (89.4) | 70 (94.6) | |
| Family history of multiple sclerosis | 0.173 | |||||
| Paternal | 248 (9.6) | 51 (9.6) | 6 (4.9) | 30 (7.4) | 6 (8.1) | |
| Maternal | 308 (11.9) | 55 (10.3) | 13 (10.7) | 31 (7.7) | 9 (12.2) | |
| Both | 65 (2.5) | 16 (3) | 0 (0) | 13 (3.2) | 0 (0) | |
| Other | 19 (0.7) | 9 (1.7) | 1 (0.8) | 4 (1) | 0 (0) | |
| None | 1944 (75.2) | 401 (75.4) | 102 (83.6) | 326 (80.7) | 59 (79.7) | |
| Family history of autoimmune disorders other than multiple sclerosis | 0.006 | |||||
| Yes | 235 (9.1) | 30 (5.6) | 12 (9.8) | 22 (5.4) | 10 (13.5) | |
| No | 2349 (90.9) | 502 (94.4) | 110 (90.2) | 382 (94.6) | 64 (86.5) |
a Values are expressed as No. (%).
b Chi-square test
5. Discussion
To the best of our knowledge, this is among the first reports investigating the sociodemographic and medical history of patients with various phenotypic subtypes of MS and comparing them with that of individuals diagnosed with CIS (i.e., the presence of MS clinical manifestations in the absence of radiological features) or RIS (i.e., the presence of MS radiological presentations without characteristic clinical patterns).
In our large population-based study, we found that patients with RRMS were remarkably younger and also had a younger age of MS onset than those with other MS phenotypic subtypes and RIS. The younger age of onset in individuals with RRMS compared to those suffering from SPMS and PPMS is not an unexpected observation, given that a substantial number of patients initially diagnosed with RRMS later progress to SPMS or PPMS. Nevertheless, the younger age of onset in RRMS compared to CIS or RIS is a matter of debate. The findings of Bergsland et al. were in line with our observation as they reported a lower age of onset among patients with RRMS than those diagnosed with CIS (21). However, Muthuraman et al. (22) and Eriksson et al. (23) found no significant difference in the age of onset.
In our study, different study groups were comparable in terms of smoking and a family history of MS. There was, however, a noticeable difference in gender distribution between the study groups, particularly in relation to primary PPMS. According to our results, a significantly higher ratio of males was observed in the PPMS subgroup compared to other MS subtypes. Based on these findings, it appears that males are more predisposed to develop PPMS than other subtypes. This finding is in line with previous studies showing a higher occurrence of PPMS among males with MS as compared to other subtypes of this autoimmune disorder (24-26).
Patients with RIS undergoing neuroimaging showed a remarkably higher rate of supratentorial lesions compared to other phenotypic subgroups, while the cerebral lesions detected in MRI were dominantly disseminated throughout the brain in other phenotypic subtypes of MS. The least prevalence of brain lesions was related to infratentorial lesions. Neuroimaging findings in other studies were compatible with ours regarding brain involvement (1, 27, 28). On the other hand, some studies have suggested the appearance of infratentorial lesions as an adverse prognostic factor predicting the progression of RIS to more advanced subtypes of MS (27) and the development of clinical symptoms (16). In contrast, others have noted that the site of lesions may have a poor predictive value for RIS progression (13, 16). Nevertheless, the emergence of an infratentorial or cervical lesion may herald RIS transformation into one of the symptomatic phenotypes of MS (29).
The highest brain plaque activity was observed among RRMS patients, while a few numbers of RIS patients had active plaques. Regarding active plaques and their association with the probability of pathologic progression and clinical attacks (30), the higher rate of active plaques in RRMS seems rational, a finding that was consistent with previous reports (31, 32). However, the pathological evaluation of active lesions shows different patterns, indicating the histological diversity of active plaques in different MS phenotypic subtypes (33). At the same time, imaging findings showed that over 75% of SPMS patients had some extent of brain atrophy, and more than 86% of them had moderate-to-high lesion loads, followed by those affected by PPMS and RRMS, respectively. On the other hand, cerebral degeneration and moderate-to-high brain lesion load were observed in a small proportion of CIS or RIS patients. Similar patterns were reported by Rojas et al. in 2015, who compared the brain volume between RIS and CIS patients (34). Another report by De Stefano et al. showed a similar size among patients with RRMS and RIS (35).
Moreover, the comparison of brain volume and brain lesion load between RIS patients and control subjects revealed insignificant differences (36). Brain atrophy is not an unusual event among CIS patients (37), and some studies have mentioned this observation as an independent predictor of the progression of CIS to more severe subtypes of MS (38). Consistent with our study, the severity of brain atrophy in CIS was milder than in other MS phenotypic subgroups (39).
The spinal neuroimaging assessment revealed insignificant differences between RIS patients and those with other MS phenotypes in terms of thoracolumbar involvement. In contrast, cervical involvement, including the number of plaques, LETM, and peripheral involvement of the cervical cord, was considerably higher among SPMS patients than individuals with other MS subtypes. Most of the patients with RIS and CIS did not have cervical cord plaques. A relatively higher rate of spinal involvement has been reported in SPMS patients in previous studies (40, 41). Also, cervical involvement has been remarkably higher in patients with RIS progressed to SPMS than in other subtypes (1). Bisulca et al. identified that the development of cervical plaques was an independent predictor of the conversion of RIS to CIS or MS (13), a hypothesis that has been opposed by some other reports (42).
In our study, the presence of comorbidities, including diabetes, hypertension, and anemia, along with MS, was not significantly different among various study groups. An insignificant association was found between a positive family (parental) history of MS and MS phenotypes and RIS. Nevertheless, the concurrent incidence of other medical conditions was notably higher among RIS and CIS patients as compared to those with other MS subtypes, an observation that requires further investigations to be confirmed. To the best of our knowledge, this is the first report on the association of MS subtypes with various comorbidities. Other studies have suggested an association between MS and other autoimmune conditions, including insulin-dependent diabetes, rheumatoid arthritis, vitiligo, and graves disease (43, 44). Furthermore, the genetic basis of MS should not be ignored, as the risk of MS is higher in a person whose parents suffer from the disease (45).
Regardless of its credibility and relevance, this study has several other strengths. High statistical power and generalizability of the findings are guaranteed by including a large sample size of 3716 patients from a single clinic. Also, a variety of MS phenotypes, including CIS, RRMS, SPMS, PPMS, and RIS, were investigated, allowing for a comprehensive comparison of sociodemographic and neuroimaging characteristics among these different subgroups. It is possible to make valuable comparisons between different phenotypes using a cross-sectional design, which provides a snapshot of the characteristics of each phenotype at one particular time point. Additionally, the incorporation of neuroimaging assessments (the location of plaques, plaque activity, brain atrophy, and lesion load) contributed to the objectivity and credibility of our findings.
It is important to take into account the limitations of this study as well when interpreting the findings. Considering the study’s single-clinic setting, there is a possibility for selection bias, meaning that the patient population in our clinic may not be representative of whole MS patients in the country. Further, due to the cross-sectional design of the study and the short time period between June 2018 and April 2019, it was inapplicable to observe long-term trends and changes in MS phenotypes over time. In addition, the differences observed between various phenotypes might have been influenced by confounding factors, such as treatment history, comorbidities, or socioeconomic status, which should be taken into account. Last but not least, despite providing useful comparisons, the study failed to establish causality or explain the mechanisms behind the observed differences. Our understanding of MS phenotypes and their characteristics can be upgraded through further research on larger and more diverse populations, designing longitudinal studies, and effectively controlling for confounding factors.
5.1. Conclusions
Besides the significant differences observed between RRMS, PPMS, and SPMS in terms of the age of onset, lesion distribution, and lesion burden, this study provided valuable insight into the clinical and imaging characteristics of different MS phenotypic subtypes. The onset of RRMS generally happened at a younger age compared to RRMS, and there was a higher frequency of supratentorial lesions and active brain plaques in RRMS patients. Moreover, SPMS and PPMS patients showed higher ratios of brain atrophy, lesion loads, cervical plaques, and longitudinally extensive transverse myelitis. The incidence of comorbidities and family history of autoimmune diseases were also higher in patients with PPMS. The results of this study can help develop more personalized approaches for managing and treating MS and can lead to a better understanding and characterization of MS phenotypes. Follow-up studies are recommended in order to identify the factors predicting the conversion of RIS and CIS to other MS phenotypes.