1. Background
Opioid use disorder is a complex disease. Genetic and environmental factors play a role in developing substance abuse disorder and relapse tendency (1). Methadone maintenance treatment (MMT) is an extensively used practice for opioid addiction disorder with proven better outcomes for adherent clients. Despite the significant health outcomes of MMT, little is known about the dynamics of methadone adherence and its determinants (2-4). Considering retention in treatment and/or not using illicit drugs as the main outcome of therapy, researchers have found that 30% to 80% of treated cases respond poorly to MMT. A reason for this wide reported range of poor responders is that no common, unique definition exists for retention in MMT, and there are different levels of tolerance for illicit drug use during MMT, from zero tolerance to acceptable functional drug use, which may be a representation of different contexts (5-7).
Several neurobiological factors are attributed to opioid use behaviors; among them, neurotrophins have a relevant role. Neurotrophic factors are associated with neuronal differentiation and growth during development and are involved in several types of plasticity in the central nervous system of adults (8). The role of glial cell line-derived neurotrophic factor (GDNF) and brain-derived neurotrophic factor (BDNF) in the modulation of addictive behaviors has been indicated by some researchers (9-11).
These two neuropeptides ensure trophic support for adult dopaminergic neurons and play a role in midbrain dopamine release regulation (12, 13). Also, they are associated with memory and learning formation and are involved in the long-term potentiation of synaptic strength, which underlies not only natural adaptation mechanisms (11, 14) but also the development of addictive behaviors (15-17), which play a critical role in drug reward (9, 18) and relapse (9, 19, 20).
It is hypothesized that long-term relapse vulnerability and compulsive drug seeking are because of drug-related neuroadaptations in the glutamatergic corticolimbic circuitry and mesocorticolimbic dopamine system, where the dopamine projections are embedded (9, 21, 22).
BDNF positively modulates psychostimulant and opiate reward within the mesocorticolimbic dopamine system. BDNF-TrkB transmission plays a role in cocaine- and morphine-related CPP in the VTA (23, 24). GDNF negatively regulates the behavioral effects of drugs of abuse because reducing endogenous GDNF levels or suppression of the GDNF pathway can increase many behavioral and biochemical adaptations to opioids, psychostimulants, and ethanol, while GDNF administered in the mesolimbic system causes opposite effects (25). According to preclinical studies, after GDNF secretion within the striatum, the molecule is retrogradely transported to the dopaminergic neurons within the mesencephalon. This retrograde GDNF signaling is assumed to indicate an inhibitory feedback mechanism that explains the inverse association between central GDNF expression and addictive behaviors (9).
Few clinical studies have investigated a putative correlation between BDNF and GDNF serum levels and substance dependence symptomatology. According to De Cid et al. (6), BDNF variability can confer differential MMT response sensitivity in patients with opioid dependence. Heberlein et al. (16) found that BDNF serum levels are linked to craving in patients with opiate dependence. According to Kotan et al. (8), GDNF serum levels are significantly lower in heroin users than in healthy people. Moreover, serum GDNF levels and anxiety, impulsivity, and depression rates are significantly associated. The possibility of using neurotrophic factors as potential biomarkers in the addiction process is an interesting topic of ongoing debate (26).
2. Objectives
Based on the mentioned studies, we designed this study to assess changes in the GDNF and BDNF serum levels in MMT adherents, MMT non-adherents, subjects who were in remission of opioid use disorder, and healthy controls. Besides, we have studied the possible association between BDNF and GDNF serum levels and opioid craving.
3. Methods
We assessed 43 opioid-dependent male patients recruited from four private methadone maintenance treatment clinics in the 11 and 12 districts of Tehran, where most residents are from the middle class. The reason for recruiting patients from these two districts was that there were more MMT clinics and DICs in these areas compared to other districts. As a result, the target of DICs was not mixed with MMT clinics (which happens in other districts where there are not adequate DICs), and patients attending MMT clinics were more homogenous in these two districts. Patients were Iranian, fulfilled diagnostic criteria for opioid use disorder based on the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5), enrolled in MMT for at least three months, not more than five months, and reported receiving a stable methadone dose for the last two months. Exclusion criteria were severe cognitive deficit, language-associated barriers, a severe psychotic disorder in the last six months, suicide attempts in the last 12 months, alcohol use disorder, methamphetamine use disorder, major cardiovascular disorder, and epilepsy. Heroin was the main substance for all the patients in the last month before participating in the methadone maintenance program. A trained psychiatrist examined all participants after signing the informed consent form.
The Tehran University of Medical Sciences (TUMS) (IR.TUMS.MEDICINE.REC.1400.352) accepted the research. The serum levels of BDNF and GDNF in 43 opioid-dependent male patients were investigated directly before consumption of the regular dose of methadone in the morning and were compared to the serum levels of BDNF and GDNF in 12 male subjects in remission of opioid use disorder and 20 healthy male controls (Table 1).
| Groups | Age (y) | Dose of Methadone Per Day (mg) | Body Mass Index |
|---|---|---|---|
| MMT clients | 41.6 ± 10.3 | 80 ± 35 | 25.1 ± 4.3 |
| Remission | 40.5 ± 10.5 | - | 25 ± 4.1 |
| Control | 39.1 ± 8.3 | - | 24.8 ± 4 |
Group Characteristics in Terms of Age, Methadone Dose, and Body Mass Index a
Craving for heroin was measured by the Persian version of the Desires for Drug Questionnaire (DDQ) (27) for three main craving components: Desire and intention, negative reinforcement, and control.
The BDNF and GDNF serum levels were assessed using the Human BDNF ELISA Kit Zell Bio GmbH (Germany) Cat. No: ZB-11302-H9648 and Human GDNF ELISA Kit Zell Bio GmbH (Germany) Cat. No: ZB-10122-H9648.
The experiments were conducted based on the producer’s directions. The lower determination thresholds were 31.3 pg/mL (BDNF) and 0.313 ng/mL (GDNF). The intra-assay and inter-assay coefficients of variation were respectively 10% and 12% (BDNF) and 10% and 12% (GDNF).
Urine tests for tracing illicit opioids were performed three months after serum sampling. Those with negative last four urinalysis tests for illicit opioids were considered MMT adherents, and non-adherents were those with one or more positive tests for illicit opioids. Failure to be present during the urine test was considered a positive test for opioid use. Urinalysis to detect opioid use was performed at clinics randomly every week under the supervision of nursing staff.
3.1. Statistical Analysis
Based on the Kolmogorov-Smirnov test, brain-derived neurotrophic factor and GDNF serum levels showed a normal distribution. Pearson's correlation coefficient assessed the correlation between the BDNF and the GDNF serum levels and the psychometric dimensions of heroin craving. Differences between the GDNF and BDNF serum levels of the opioid-dependent patients, subjects in remission, and the healthy control group were analyzed by ANOVA. Data were analyzed using SPSS 18 and Minitab 16.
4. Results
Overall, 43 male patients on MMT, 12 male subjects in remission from opioid use disorder, and 20 male healthy controls were enrolled. The participants’ mean age was 40.40 ± 10.11 years. For the MMT patients, the mean age was 41.6 ± 10.3 years, the average body mass index was 25.1 ± 4.3, and the mean dose of methadone per day was 80 ± 35 mg. For the people in remission, the mean age was 40.5 ± 10.5 years, and the average body mass index was 25 ± 4.1. For the healthy controls, the mean age was 39.1 ± 8.3 years, and the average body mass index was 24.8 ± 4. The average age and body mass index (BMI) of each group and the amount of methadone received by the treated group are given in Table 1.
The differences between BMI and age in the three groups were not statistically significant. This means that matching the groups has been implemented, and the possibility of the influence of these two variables on the research findings is very low.
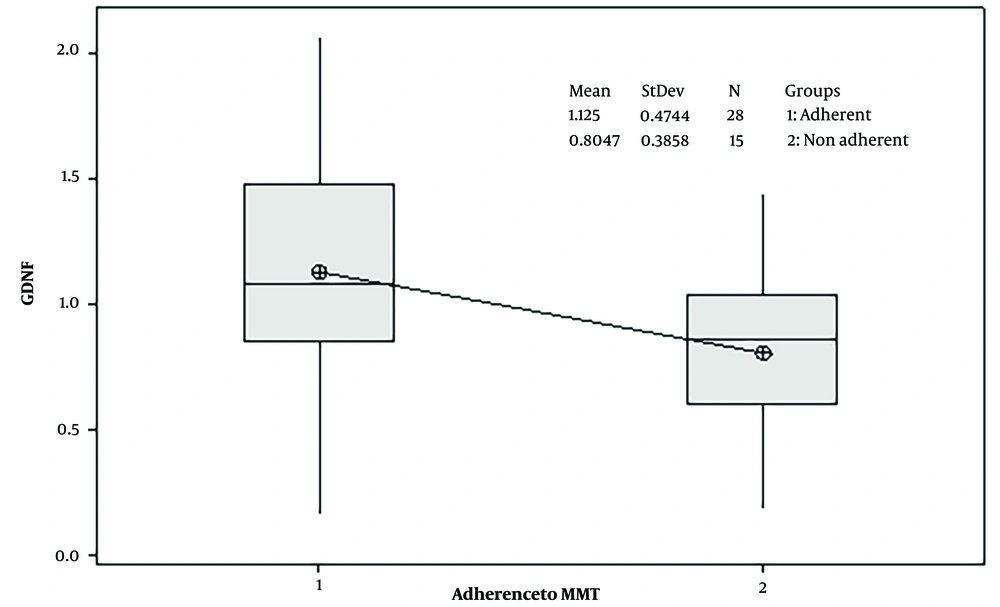
Using a t-test for independent groups, the difference in serum levels of GDNF between adherent patients to MMT (28 subjects) and non-adherent patients (15 subjects) was significant (P-value = 0.03). Adherent subjects had higher levels of GDNF (mean ± SD: 1.125 ± 0.4744) compared to the non-adherent subjects (mean ± SD: 0.8047 ± 0.3858) (the threshold of significance = 0.05). These findings are shown in Figure 1.
Regarding serum levels of BDNF, the difference between adherent and non-adherent subjects was not statistically significant (P-value = 0.08).
The ANOVA test compared variations of the BDNF and GDNF serum levels amongst MMT clients, people in remission, and healthy control groups, and no significant differences were found (P-value = 0.13). There were no significant correlations between the variations in the BDNF (P-value = 0.13) and GDNF (P-value = 0.59) serum levels and the heroin craving psychometric dimensions.
5. Discussion
We found that GDNF variability can be involved in MMT adherence. Adherent patients had higher serum levels of GDNF compared to non-adherent patients. The BDNF serum levels were not significantly different between adherents and non-adherent patients. Although the association between BDNF and GDNF and opioid dependence disorder has been documented (6, 8, 16, 28-31), to our knowledge, this is the first time that GDNF variability has been associated with adherence to MMT.
Our results regarding GDNF align with previous preclinical research, indicating GDNF's role as a negative regulator of drug intake. According to preclinical studies, after GDNF secretion within the striatum, it is retrogradely transported to the dopaminergic neurons within the mesencephalon. This retrograde GDNF signaling is assumed to indicate a negative feedback mechanism that illustrates the negative association between central GDNF expression and addictive behavior. Several studies have reported increased self-administration and conditioned place preference in animals following the intake of substances such as morphine, cocaine, alcohol, and methamphetamine when GDNF function was reduced (9). Preclinical studies have also shown that long-term exposure to cocaine and morphine significantly reduces central GDNF signaling (32).
In a study by Kotan et al. (8) on GDNF levels in heroin addicts, the research team considered GDNF because of its more potent effects on the protection and survival of dopaminergic neurons than BDNF and other neurotrophins. Sensation seeking, impulsivity, and behavioral control as traits related to the extensive construct of disinhibition predict substance use disorders in adults and are higher in substance-dependent patients (33, 34). It is claimed that people who use substances are more impulsive (35, 36); however, the mechanism of this impulsivity has not yet been identified.
The lower GDNF serum levels in non-adherent MMT patients in our study were consistent with those of Kotan et al. (8). They found that people with heroin use disorder had lower GDNF levels than healthy controls. Moreover, they showed that decreased GDNF serum levels in people with heroin use disorder are associated with anxiety, impulsivity, and depressive symptoms. We did not find an association between BDNF and GDNF serum levels and opioid craving. The differences in the types of subjects enrolled in studies could be a reason for disagreements between results, as the subjects involved in the study by Kotan and colleagues were people with heroin use disorder and healthy controls. However, the subjects in our study were patients who were stabilized on methadone maintenance treatment, healthy controls, and people in remission.
Regarding BDNF, Ghitza et al. (9), in their review of preclinical studies, indicated that BDNF is a positive modulator of psychostimulant and opioid reward within the mesocorticolimbic dopamine system. In contrast to this review, Koo et al. (37) in their study indicated BDNF as a negative modulator of morphine action. They observed that the mechanisms of neuroadaptation that regulate reward by opioids can be different and even opposite to those that promote reward by stimulant drugs. Heberlein et al. (16) reported an increase in BDNF serum levels in opiate-dependent cases during opiate maintenance compared with healthy controls. Similarly, according to De Cid et al. (6), BDNF variability can be involved in the response to MMT independently of personality traits, environmental cues, methadone dosage, and psychiatric comorbidity. Contrary to these studies, we detected no significant difference in the BDNF serum levels of MMT adherents, non-adherent patients, subjects in remission of opioid use disorder, or healthy controls. The discrepancy in the results observed in our study and the others could be related to the different medications with different pharmacokinetic and pharmacodynamic characteristics used as the maintenance treatment for opioid use disorder. For example, the patients in the study by Heberlein and colleagues have received diacetylmorphine (DAM) injections as the maintenance medication. The half-life of DAM is shorter than that of methadone, making patients attend the clinic twice daily to receive DAM. However, our patients received methadone with a longer half-life as their maintenance medication, and they were stabilized on their dosage for at least two months. More studies are needed to completely define the effect of different maintenance medications on patients' BDNF or GDNF serum levels. In their review, Ghitza et al. (9) concluded that whether GDNF or BDNF would suppress or facilitate drug use behaviors is associated with the brain site, the drug type, and the addiction phase (maintenance, initiation, or abstinence/relapse). Our study, which implies lower GDNF serum levels in non-adherent MMT patients compared to adherent ones, emphasized the importance of considering these sample differences when interpreting the results.
Our small sample size and involvement of only male Iranian subjects in the study would limit the results' generalizability to females or other ethnic or demographic groups. Thus, future investigations using a larger, more general sample and considering pharmacokinetic factors affecting the methadone treatment response are needed. Moreover, our study was cross-sectional and unable to explain the cause-and-effect relationship between GDNF and opioid use disorder. The other limitation of our study was the possibility of influence by third-class factors that we didn't consider, like depressed mood and nicotine consumption. In addition, other potential confounding variables, such as dietary habits, sleep patterns, or other lifestyle factors, might influence serum levels of BDNF and GDNF and should be investigated in future studies.
5.1. Conclusions
Our results suggest GDNF's involvement as a neurobiological factor in adherence to MMT. Adherent patients had higher serum levels of GDNF compared to the non-adherent subjects. The BDNF serum levels were not significantly different between adherents and non-adherent patients. We couldn’t find an association between BDNF and GDNF serum levels and opioid craving. Future investigations using a larger sample should be performed and consider pharmacokinetic factors affecting the methadone treatment response. The contribution of other potential confounding variables that might influence serum levels of BDNF and GDNF, like nicotine consumption, psychostimulants, mood disorders, antidepressant treatment, and the association between impulsivity scores and cravings, can be investigated in the future.