1. Background
Delirium is an acute disturbance in consciousness characterized by a decline in attention and cognitive impairment. The common clinical manifestations of delirium include memory deficits, fluctuating levels of awareness, hallucinations, disorientation, and movement disorder (1). Various risk factors contribute to the development of delirium, including depression, dementia, alcohol use, smoking, and especially the process of aging (2, 3).
The prevalence of delirium among hospitalized elderly patients can reach up to 30% (4, 5). Although delirium can manifest at any age, elderly individuals with permanent and progressive brain damage, cerebrovascular disease, or head trauma, especially those experiencing cognitive dysfunction, are particularly vulnerable to delirium during periods of biological stress (6, 7). Among the elderly, depression stands out as one of the most significant risk factors for delirium, with prevalence estimates ranging from 8% to 16% among adults aged 60 years and above (8, 9). The pathophysiology of depression involves the impairment of serotonin pathways. Several studies have demonstrated that ketamine has the ability to enhance increased serotonin release and upregulate serotonergic receptors in brain regions associated with depression (10-12).
Delirium is common in the intensive care unit (ICU) population and is associated with multiple detrimental consequences. The mortality rate in ICU patients who suffer from delirium is three times higher than in other patients (13). Delirium is notably significant in ICU patients, particularly in the elderly population, due to several reasons. Delirium is a common occurrence in ICU patients, and its prevalence is higher in older individuals. The elderly population is particularly vulnerable to delirium due to age-related changes in the brain, higher rates of comorbidities, and increased susceptibility to physiological stressors. The presence of delirium is associated with worse outcomes, including prolonged hospital stays, increased risk of complications, functional decline, and higher mortality rates (14).
Delirium can be an early warning sign of underlying physiological or neurological deterioration, making its recognition and management crucial for optimizing patient care. Delirium in the ICU can result in long-lasting cognitive impairment, particularly in the elderly. Older patients who experience delirium are at a higher risk of developing persistent cognitive deficits, including memory problems, attention deficits, and executive dysfunction. These cognitive impairments can have a profound impact on patients’ quality of life, functional independence, and ability to return to their pre-ICU level of functioning. Delirium can contribute to functional decline in ICU patients, especially in the elderly population. Patients with delirium might experience difficulties with mobility, self-care, and activities of daily living. This functional decline can prolong hospital stays, increase the need for rehabilitation services, and impact patients’ ability to return to their pre-hospitalization level of function.
Delirium in ICU patients, particularly in the elderly, is associated with increased healthcare utilization and costs. Patients with delirium often require longer ICU and hospital stays, additional diagnostic tests, increased use of medications, and rehabilitation services (15, 16). Given the substantial impact of delirium on the health outcomes, functional status, cognition, and overall well-being of ICU patients, especially the elderly, there is a pressing need for early recognition, prevention, and effective management strategies (17, 18).
Current strategies for preventing delirium include the use of first-generation antipsychotics (e.g., haloperidol, chlorpromazine, and droperidol), second-generation antipsychotics (e.g., loxapine, ziprasidone, and olanzapine), benzodiazepines (e.g., lorazepam, diazepam, and midazolam), and more recently, ketamine (19-23). However, studies investigating the effectiveness of pharmacological interventions for controlling delirium have shown conflicting outcomes (24).
Ketamine, an intravenous anesthetic, is increasingly being used as an alternative agent to treat symptoms of agitation and delirium (19, 25, 26). Emerging preclinical and clinical research has shown the neuroprotective properties of ketamine (27). A small clinical trial involving cardiac surgery patients showed that administering a low dose of ketamine significantly reduced postoperative delirium without causing any noticeable side effects (28). Moreover, the administration of low-dose ketamine was shown to improve postoperative cognitive impairment following cardiac surgery (29). Furthermore, ketamine has been found to reduce the incidence of emergency delirium in children (30). Ketamine is a safe, fast-acting, and effective treatment for reducing delirium, possibly due to its neuroprotective effects (31). Additionally, ketamine is cost-effective and has been extensively utilized by anesthetists for over six decades. Although there is growing evidence supporting the use of ketamine in delirium prevention, studies are limited, and the current research has not definitively established its efficacy in older hospitalized patients (32).
There are few studies investigating the efficacy of ketamine as a first-line treatment for elderly patients with delirium (33, 34). The present study aimed to investigate the safety and efficacy of administering low-dose ketamine as a potential treatment for reducing delirium in elderly ICU patients. The current study hypothesized that the use of ketamine would be effective in controlling delirium symptoms when compared to the commonly used medication haloperidol. By conducting a randomized clinical trial, this study sought to evaluate the hypothesis and provide insights into the potential benefits of ketamine as a first-line treatment for delirium in elderly patients.
2. Methods
2.1. Study Design
This open-label randomized clinical trial enrolled non-intubated elderly patients aged 65 years and above who were admitted to the ICU of Imam Khomeini Hospital Complex, Tehran, Iran. Patients were recruited between April 2020 and March 2021 at the Imam Khomeini Hospital Complex, Tehran University of Medical Sciences, Tehran, Iran, and obtained approval from the Ethics Committee of Tehran University of Medical Sciences. Before participating in the study, eligible individuals or their representatives provided written informed consent.
The inclusion criteria for this study were patients aged over 65 years admitted to the ICU, with a Richmond Agitation Sedation Scale (RASS) score equal to or more than 2 and non-positive for troponin and B-type natriuretic peptide (BNP). The patients’ systolic pressure had to be less than 140 mm Hg, diastolic pressure less than 95 mm Hg, and heart rate less than 100 bpm. Patients with a history of psychosis, including symptoms such as hallucinations and delusions, a documented diagnosis of schizophrenia, and a history of disorganized thinking and impaired functioning, were excluded from the study. In addition, individuals with a history of cardiovascular diseases, including coronary artery disease, myocardial infarction, heart failure, arrhythmias, and stroke, were also excluded from participation.
2.2. Randomization
A blocked randomization method was employed to assign 44 patients in a 1: 1 ratio to either the low-dose ketamine or haloperidol group. Upon admission, all patients were randomly allocated to one of two groups: The ketamine group, which received 20 mg of intravenous ketamine, or the haloperidol group, which received 2.5 mg of intramuscular haloperidol. Randomization was performed using random number tables. To generate the random number tables, an online random number generator (https://www.graphpad.com/quickcalcs/randomize1.cfm) was utilized. The mentioned number generator was employed to generate a sequence of random numbers. These random numbers were then used to assign the participants to different treatment groups.
2.3. Study Procedures
Patient interviews and delirium assessments were conducted by experienced clinicians and researchers. Baseline data, including questionnaires and demographic information, such as age, gender, and education, were recorded. The RASS was used to assess the severity of delirium in ICU patients. Levels of alertness, sedation, and agitation were evaluated at 5, 10, and 15 minutes following the intervention. Any side effects and physician satisfaction regarding the control of agitation (rated as weak, moderate, good, or excellent) were recorded in the patients’ checklist at 60 minutes. Adequate agitation control was considered present if the responses were rated as good or excellent.
To evaluate delirium, the first step was observation. A score of 0 to +4 was considered for conscious patients, with +4, +3, +2, and +1 representing aggressive, restless, agitated, and irritable patients, respectively. For patients who were not conscious or alert, the second step was verbal stimulation by calling their names loudly. A score of -1 to -3 was given if the patient reacted to the verbal stimulation. If the patient did not show any reaction to verbal stimulation or it was insufficient, the next step was physical stimulation, which was assigned a score of -4 to -5.
2.4. Study Outcomes
The criterion for recovery and adequate sedation was defined as a RASS score of 1 or less. The primary outcome was the percentage of patients who achieved adequate sedation; however, the secondary outcome was the time needed to reach adequate sedation. To evaluate possible side effects, information, such as changes in QT interval, blood pressure, heart rate, seizures, and hallucinations, was recorded before and after the interventions.
2.5. Statistical Analysis
Statistical analyses were performed using GraphPad Prism software (version 8). The numerical data were analyzed using t-tests and repeated-measures analysis of variance (ANOVA). For the comparison of age, clinical characteristics (blood pressure, heart rate, respiratory rate, and oxygen saturation), RASS score, and delirium incidence between the two groups, a t-test was employed. To compare the RASS scores at different time points in the ketamine and haloperidol groups, repeated-measures ANOVA was used. The chi-square test was utilized to compare gender, education, marital status, and physician satisfaction as categorical variables between the two groups. The data are presented as mean ± standard deviation (SD), and P-values less than 0.05 were considered statistically significant.
3. Results
3.1. Study Patients
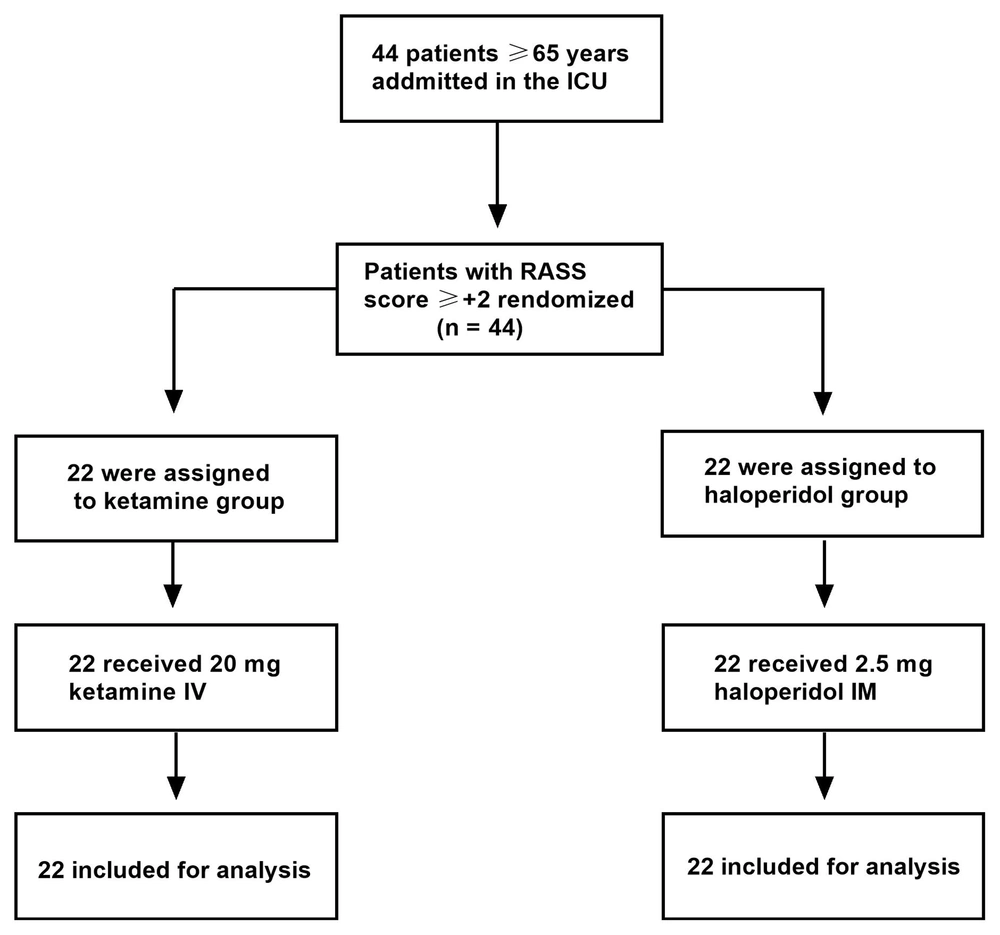
A total of patients were randomly assigned to either the ketamine group (n = 22) or the haloperidol group (n = 22) (Figure 1). There were no statistically significant differences between the two groups in terms of demographic characteristics, such as age (P = 0.553), gender (P = 0.741), marital status (P = 0.750), and education (P = 0.822) (Table 1).
| Ketamine (n = 22) | Haloperidol (n = 22) | P-Value | |
|---|---|---|---|
| Age (y), mean± SD | 69.2 ±95.60 | 70.2 ± 45.92 | 0.553 |
| Gender | 0.741 | ||
| Female | 15 (68.2) | 6 (27.3) | |
| Male | 7 (31.8) | 16 (72.7) | |
| Married | 14 (63.6) | 15 (68.2) | 0.750 |
| Education (y) | 0.822 | ||
| ≤ 6 | 8 (36 4) | 10 (45.5) | |
| 6 - 12 | 9 (40.9) | 8 (36 4) | |
| ≥ 12 | 5 (22.7) | 4 (18.2) |
Abbreviation: SD, standard deviation.
aValues are expressed as No. (%) unless otherwise indicated.
There were no significant differences between the effects of ketamine and haloperidol on blood pressure, heart rate, respiratory rate, or oxygen saturation at different time points (Table 2). The p-value comparing the groups was 0.278 (effect size = 0.028) for blood pressure, 0.392 (effect size = 0.017) for heart rate, 0.961 (effect size = 0.00) for respiratory rate, and 0.301 (effect size = 0.026) for oxygen saturation, and the p-value within each group was not significant for all variables (blood pressure = 0.338, effect size = 0.025; heart rate = 0.580, effect size = 0.012; respiratory rate = 0.335, effect size = 0.024; oxygen saturation = 0.467, effect size = 0.019).
| Clinical Characteristics | 0 min | 5 min | 10 min | 15 min |
|---|---|---|---|---|
| Blood pressure | ||||
| Ketamine | 121.45 ± 8.93 | 124.32 ± 12.27 | 123.18 ± 10.52 | 123.64 ± 10.52 |
| Haloperidol | 121.36 ± 8.75 | 120.68 ± 8.63 | 116.59 ± 21.34 | 122.73 ± 5.28 |
| Heart rate | ||||
| Ketamine | 80.86 ± 9.23 | 81.32 ± 6.94 | 80.91 ± 4.92 | 81.14 ± 4.4 |
| Haloperidol | 78.23 ± 9.37 | 79.32 ± 8.87 | 79.95 ± 6.91 | 80.27 ± 6.81 |
| Respiratory rate | ||||
| Ketamine | 17.50 ± 1.71 | 17.59 ± 1.50 | 17.40 ± | 17.05 ± 1.65 |
| Haloperidol | 17.45 ± 2.11 | 17.45 ± 1.53 | 17.86 ± 1.08 | 17.63 ± 1.32 |
| Oxygen saturation | ||||
| Ketamine | 97.36 ± 1.7 | 97.73 ± 1.8 | 97.86 ± 1.58 | 97.68 ± 1.58 |
| Haloperidol | 98.05 ± 1.49 | 98.38 ± 0.97 | 97.81 ± 1.46 | 98.09 ± 1.63 |
aValues are expressed as mean ± SD.
3.2. Study Outcomes
There was no significant difference between the two groups in terms of delirium score (RASS score) after receiving the intervention, as shown in Table 3. The p-value comparing the groups was 0.168 (effect size = 0.042), indicating no significant difference. However, the P-value within each group was < 0.001 (effect size = 0.601), indicating a significant difference.
| Group | 0 min | 5 min | 10 min | 15 min |
|---|---|---|---|---|
| Ketamine | 2.77 ± 0.75 | 0.27 ± 1.27 | 0.31 ± 1.39 | -0.36 ± 1.62 |
| Haloperidol | 2.77 ± 0.75 | 1.5 ± 1.68 | 0.59 ± 1.95 | 0.13 ± 1.95 |
aValues are expressed as Mean ± SD.
In the subsequent analysis, the incidence of delirium was evaluated by assessing the percentage of patients whose RASS score decreased to 1 or lower (Table 4). Five minutes after the intervention, based on the RASS score, 86.4% of patients in the ketamine group achieved satisfactory sedation (RASS ≤ +1); nevertheless, only 36.4% in the haloperidol group achieved the same level of sedation. A significant difference was observed between the two groups (P = 0.002). At the 10-minute mark, no significant difference was observed between the two groups (P = 0.082), with 86.4% and 63.3% of patients in the ketamine and haloperidol groups achieving RASS scores of 1 or less, respectively. Similarly, at the 15-minute mark, 86.4% and 63.3% of patients in the ketamine and haloperidol groups, respectively, achieved adequate sedation (RASS ≤ +1); however, no statistically significant difference was observed between the two groups (P = 0.082).
| RASS Score | Ketamine (n = 22) | Haloperidol (n = 22) | P-Value |
|---|---|---|---|
| 5 min | 0.002 | ||
| ≤ +1 | 19 (86.4) | 8 (36.4) | |
| > +1 | 3 (13.6) | 14 (63.6) | |
| 10 min | 0.082 | ||
| ≤ +1 | 19 (86.4) | 14 (63.6) | |
| > +1 | 3 (13.6) | 8 (36.4) | |
| 15 min | 0.082 | ||
| ≤ +1 | 19 (86.4) | 14 (63.6) | |
| > +1 | 3 (13.6) | 8 (36.4) |
Abbreviation: RASS, Richmond Agitation Sedation Scale.
aValues are expressed as No. (%).
To evaluate physician satisfaction, 13.6% and 18.2% of patients in the ketamine and haloperidol groups had weak satisfaction, respectively; however, 13.6% and 36.4% of patients in the ketamine and haloperidol groups had moderate satisfaction, respectively. Additionally, 18.2% and 22.7% of patients in the ketamine and haloperidol groups had good satisfaction, respectively; nonetheless, 54.5% and 22.7% of patients in the ketamine and haloperidol groups had excellent satisfaction, respectively. There was no significant difference between the two groups in terms of physician satisfaction (P = 0.144). Physician satisfaction is presented in Table 5.
| Physician Satisfaction | Ketamine (n = 22) | Haloperidol (n = 22) | P-Value |
|---|---|---|---|
| Weak | 3 (13.6) | 4 (18.2) | 0.144 |
| Moderate | 3 (13.6) | 8 (36.4) | |
| Good | 4 (18.2) | 5 (22.7) | |
| Excellent | 12 (54.5) | 5 (22.7) |
aValues are expressed as No. (%).
4. Discussion
Managing delirium is a vital objective when caring for ICU patients, particularly the elderly population. Prolonged delirium is associated with elevated mortality rates and an increased likelihood of hospital readmissions (18, 35). Previous studies have shown that brain volume and weight decreased with age (36-38), and structural and functional changes in brain regions, such as the hippocampus, have been reported in older populations (39-42). As a result, elderly patients experience a higher occurrence of delirium than other populations. Moreover, patients with delirium lasting less than 24 hours tend to have similar outcomes to those without delirium (43). Therefore, the present study evaluated delirium in patients over 65 years.
This open-label randomized clinical trial compared the safety and efficacy of low-dose ketamine to haloperidol in the incidence and control of delirium among elderly ICU patients. The study showed no significant difference between the two treatments in terms of delirium (based on RASS scores) at different times (5, 10, and 15 minutes) after the intervention in older patients. This finding suggests that both ketamine and haloperidol had a similar effect on delirium in this specific population. These findings indicate that when it comes to the primary outcome of reducing delirium, there was no advantage of using one medication over the other.
Additionally, the present study did not show any significant side effects associated with either ketamine or haloperidol treatment. The absence of significant side effects observed in the ketamine group is an important finding, indicating that low-dose ketamine was well tolerated by the elderly ICU patients in the trial. This is a positive outcome as it suggests that this medication can be safely used in this context without causing significant adverse effects. Therefore, ketamine can be considered a safe option for managing delirium in this specific patient group.
On the other hand, the study demonstrated a significant reduction in RASS scores within each treatment group at 5, 10, and 15 minutes, compared to the baseline of 0 minutes. This reduction in RASS scores indicated that low-dose ketamine was effective in reducing delirium among these patients. Therefore, the study concludes that ketamine was successful in achieving the desired outcome of reducing delirium severity in elderly ICU patients. Overall, based on the aforementioned findings, the study suggests that low-dose ketamine and haloperidol have similar efficacy and safety profiles in the prevention and management of delirium among elderly ICU patients, and these results suggest that low-dose ketamine can be considered a viable option for the control of delirium in elderly ICU patients. The present study’s findings contrast with those of Heydari et al.’s randomized clinical trial conducted in 2018. The aforementioned study demonstrated that the time required to achieve adequate sedation was significantly longer in the haloperidol group (2.5 mg) than in the ketamine group (4 mg/kg). However, there was no difference in sedation scores between the two groups 15 minutes after the intervention (44).
Additionally, in the present study, physician satisfaction with delirium control was similar between the two groups. In contrast, Heydari et al.’s study reported significantly higher physician satisfaction in the ketamine group. Consistent with the present study’s results, no significant side effects were observed between the two groups. The different outcomes between the present study and Heydari et al.’s study might be due to differences in study protocols, such as different treatment doses. In the current study, a dose of 2.5 mg haloperidol was administered; nevertheless, Heydari et al. used 5 mg. Furthermore, the present study focused on evaluating delirium in elderly ICU patients; however, Heydari et al. assessed severely agitated patients in the emergency department (44).
Apart from evaluating the time taken to achieve adequate sedation, assessing the incidence of delirium is also essential in evaluating the efficacy of delirium treatments. Therefore, the present study evaluated the percentage of patients with RASS scores equal to or less than 1. The current study’s results showed that the percentage of patients with adequate sedation (RASS ≤ +1) in the ketamine group was significantly higher than in the haloperidol group at 5 minutes following the intervention. This finding suggests that ketamine was more effective in achieving the desired level of sedation at the earlier time point. However, at 10 and 15 minutes after the intervention, there was no significant difference observed between the ketamine and haloperidol groups regarding the percentage of patients with adequate sedation. This finding indicates that both ketamine and haloperidol had similar efficacy in achieving and maintaining adequate sedation at these later time points. The aforementioned findings suggest that although ketamine might provide a faster onset of sedation than haloperidol at 5 minutes, there is no significant advantage in terms of achieving and maintaining adequate sedation between the two medications at later time points.
The above-mentioned findings are consistent with the findings of a previous pilot study on ketamine for postoperative delirium prevention in spinal fusion surgery, which also showed that the incidence of delirium differed between the pre-implementation and post-implementation groups at arrival, 60, and 90 minutes after surgery. Ketamine reduced the incidence of delirium in the initial 90 minutes (45). Previous placebo-controlled clinical trials demonstrated that low doses of ketamine (2 mg/kg/h) can reduce the duration and incidence of postoperative delirium in general ICU patients (46). However, an international multicentre clinical trial on older adults showed that intraoperative ketamine did not prevent postoperative delirium after major surgery. There were no significant differences in the delirium incidence between the ketamine groups and the placebo group, and neither the low dose (0.5 mg/kg) nor the high dose (1 mg/kg) of ketamine reduced the incidence of delirium in patients over 60 years of age (34).
Disturbance in the synthesis and release of neurotransmitters, such as reduced cholinergic and serotonergic activity and excess release of glutamate, can lead to functional connectivity alteration of neurons, resulting in atrophy and brain damage, which play crucial roles in delirium development (47-51). Ketamine has been shown to balance these neurotransmitter releases in the brain (52-54). Moreover, the beneficial effect of ketamine on preventing delirium might be due to its anti-inflammatory properties (27), neuroprotective activity (55), and anti-depressant properties (56). A study by Hudetz et al. on elderly patients undergoing cardiac surgery showed that the injection of 0.5 mg/kg of ketamine during the anesthesia decreased the incidence of postoperative delirium. The reduction in delirium was associated with a decrease in serum C-reactive protein (CRP) concentrations, suggesting the anti-inflammatory function of ketamine (29).
Inflammation plays a significant role in the development of delirium, as indicated by previous studies (57, 58). Surgical trauma, for instance, can trigger an elevation in inflammatory markers, such as CRP, which can subsequently promote the generation of reactive oxygen species (ROS). These ROS have the potential to induce disruption of the blood-brain barrier (BBB) and contribute to the onset of delirium (57). Ketamine’s anti-inflammatory properties might contribute to its potential in reducing delirium through several mechanisms. Ketamine has been shown to inhibit the production and release of pro-inflammatory cytokines, such as interleukin-1 beta (IL-1β), interleukin 6 (IL-6), and tumor necrosis factor-alpha (TNF-α). These cytokines are known to play a role in promoting neuroinflammation and can contribute to the development or worsening of delirium. By reducing the levels of pro-inflammatory cytokines, ketamine might help mitigate the inflammatory response associated with delirium (59, 60).
Microglia, the immune cells of the central nervous system, play a crucial role in neuroinflammation. Ketamine has been observed to modulate the activation of microglia, suppressing their pro-inflammatory response. By inhibiting excessive microglial activation, ketamine might help attenuate neuroinflammation and potentially alleviate delirium symptoms (61). Inflammatory processes can generate ROS and lead to oxidative stress, which can damage neurons and contribute to cognitive impairment seen in delirium. Ketamine has been shown to possess antioxidant properties, effectively scavenging ROS and reducing oxidative stress. By reducing oxidative damage, ketamine might protect against neuronal injury and potentially mitigate delirium symptoms (62-64).
The BBB is a protective barrier that regulates the passage of substances into the brain. Inflammatory processes can compromise BBB integrity, allowing the entry of inflammatory mediators into the brain. Ketamine has been observed to help preserve BBB integrity by inhibiting the disruption of tight junction proteins. By maintaining BBB integrity, ketamine might help prevent the infiltration of pro-inflammatory substances into the brain and reduce neuroinflammation associated with delirium (65). It is important to note that although ketamine’s anti-inflammatory properties are promising, further research is needed to fully understand the extent of its impact on delirium. Additionally, the anti-inflammatory effects of ketamine might interact with other mechanisms of action to collectively contribute to its potential in reducing delirium.
In summary, the present trial demonstrated that low-dose ketamine and haloperidol had similar effects on delirium reduction among elderly ICU patients. Overall, these results suggest that low-dose ketamine can be considered a viable option for the incidence and control of delirium in elderly ICU patients. The study’s findings provide valuable evidence for clinicians when deciding which medication to use in this specific patient population, considering factors such as patient characteristics, contraindications, and individual treatment goals. However, it is important to note that further research and larger-scale studies are necessary to validate the aforementioned findings and provide further conclusive evidence regarding the safety and efficacy of these treatments for delirium management in elderly ICU patients.
The current study has several limitations that need to be addressed. One limitation is the small sample size, which was attributed to strict exclusion criteria and limited financial resources. A small sample size reduces the statistical power of the study and increases the likelihood of chance findings. It also limits the generalizability of the results, as the findings might not be representative of the broader population of elderly ICU patients. To overcome this limitation, future studies should aim for larger sample sizes to obtain more robust and generalizable results.
Furthermore, the study excluded patients with substantial cognitive dysfunction. This exclusion criterion might have introduced selection bias, as patients with cognitive dysfunction are more likely to develop delirium and could potentially respond differently to the studied treatments. By excluding these patients, the generalizability of the findings to the broader population of elderly ICU patients, including those with cognitive dysfunction, is limited. Future studies should consider including a wider range of patients to improve the external validity of the results.
Additionally, the study only assessed short-term outcomes at 5, 10, and 15 minutes after the intervention. Delirium is a complex condition that can have variable pathways and durations. Assessing delirium outcomes over a longer period, such as hours or days after intervention, would provide a more comprehensive understanding of the efficacy and durability of the treatments. Long-term follow-up would also be important to evaluate potential adverse effects and to assess whether the observed reduction in delirium has any impact on patient outcomes and clinical management.
Moreover, the study used an open-label design, which means that both the researchers and participants were aware of the administered treatment. This lack of blinding introduces the potential for bias in the assessment of outcomes.
4.1. Conclusions
In this study comparing low-dose ketamine to haloperidol for delirium control in elderly ICU patients, no significant difference was observed between the two treatments in terms of delirium at different time points after the intervention. However, both treatments resulted in a significant reduction in delirium scores in each group compared to the baseline. This finding indicates that low-dose ketamine effectively reduced delirium in these patients. Furthermore, both ketamine and haloperidol were well-tolerated without significant side effects.
Assessing the incidence of delirium and the time required for adequate sedation is crucial for evaluating treatment efficacy. The study showed that the percentage of patients with adequate sedation in the ketamine group was significantly higher than in the haloperidol group at 5 minutes after the intervention, suggesting that ketamine achieved the desired sedation level more quickly. However, there was no significant difference in physician satisfaction between the two groups.
The above-mentioned findings have important clinical relevance, indicating that low-dose ketamine can be a viable alternative for managing delirium in elderly ICU patients. Ketamine was shown to effectively reduce delirium, was well-tolerated, and achieved faster sedation than haloperidol. This study provides valuable insights for clinicians in choosing appropriate treatments for delirium control in this patient population. However, further studies are required to confirm the safety and efficacy of ketamine in older patients.