1. Background
Depression is one of the most common psychiatric disorders worldwide and causes disability for a lifetime (1). Despite the major prevalence rate of depression and increasing efforts to produce knowledge and skills, this disorder is still underdiagnosed and undertreated (2). The current information and treatments are not adequate, and it is required to produce new agents for depression treatment (3). A broad spectrum of pharmacotherapy agents (e.g., fluoxetine) used for the treatment of depression is based on the modulation of brain monoamines (4). Several biochemical theories have suggested imbalances in the levels of biogenic amines, such as dopamine (DA) and serotonin (5-HT), as major causes of psychiatric disorders such as depression (5, 6).
The imbalances in the dopaminergic and serotonergic systems influence the chemical balance within the entire neurotransmitter system (7). Although some studies have confirmed the efficiency of conventional treatments for depression, other studies could not prove the long-term advantages of such medications in depressed patients (8). There is a need to produce new agents for the treatment of depression with the maximum efficiency and the lowest side effects. Herbal medicine is one of the common options used for the treatment of major depressive disorder (9).
Pistacia lentiscus is a wild-growing plant containing a high content of polyphenols, which makes them attractive against chronic and degenerative diseases and also their use as a nutraceutical in human health (10, 11). It is a source of phenolic compounds with the capability to be neuroprotective due to its antioxidant activity (12). The positive effects of P. lentiscus have been reported on the central nervous system and different disorders (13). Moreover, earlier studies demonstrate the antidepressant potential of polyphenols (14, 15). However, the effect of P. lentiscus on depression has not been elucidated. To elucidate the possible mechanisms involved in depression, different types of animal models of depression are used in laboratory experiments (16).
2. Objectives
This study evaluated the antidepressant-like effect of the ethanolic extract of P. lentiscus (EEPL) in tail suspension test (TST) as a mouse model and its possible mechanisms.
3. Methods
3.1. Drugs
SCH-23390, WAY-100135, ketanserin, p-chlorophenylalanine, propranolol, and haloperidol were purchased from Sigma Co. (USA). Yohimbine and prazosin were prepared by Amin Pharmaceutical Co. (Iran). Imipramine and fluoxetine were purchased from Sobhan Darou Co. (Iran). All the agents were dissolved in normal saline plus dimethyl sulfoxide (DMSO) (5%) and administered intraperitoneally (i.p.) at a constant volume of 10 mL/kg (0.2 mL per 20 g weight of mouse).
3.2. The Preparation of Ethanolic Extract of Pistacia lentiscus
Pistacia lentiscus was prepared at a local market in Tabriz, Iran. The EEPL was prepared as reported in a previous study (17). Therefore, the freshly prepared plant was allowed to dry in the open air and at an ambient temperature in the laboratory. After drying, the aerial part was ground with a mechanical mixer to obtain a fine powder. The EEPL was obtained using the Soxhlet method, which enables a solid-liquid extraction with high quality and efficiency. For this purpose, the dried whole plant was crushed and reduced to a fine powder; then, 40 g of the powder obtained was mixed with 400 mL of a solvent (ethanol 80%) in a reflux condenser for 6 hours. After extraction, the solvent-rich substance extracted was recovered in a ball and then passed to the rotary apparatus (40°C) to evaporate the solvent and then lyophilized. Finally, the obtained extract was placed in a desiccator and was stored at 5°C until use.
3.3. Animals
Adult male NMRI mice with a weight of 20 - 25 g were prepared from Razi Institute (Tehran, Iran) and kept under 12 h L: 12 h D at room temperature. The mice had ad libitum access to water and standard rodent food.
3.4. The Tail Suspension Test
For the tail suspension test (TST), the mice were suspended 50 cm above the floor with the help of adhesive tape placed 2 cm from the tip of the tail. Immobility times were considered and recorded for a 6-minute period. Similar to the forced swim test (FST), this animal model is the best-validated test for the assessment of the acute antidepressant potential of drugs or medicinal plants and is used to evaluate the effects of genetic, neurobiological, and environmental manipulations. In contrast to FST, in the TST, there is no risk of hypothermia due to the submersion of mice in water, and this test was suggested to have a greater pharmacological sensitivity (16, 18).
3.5. Open-Field Test (OFT)
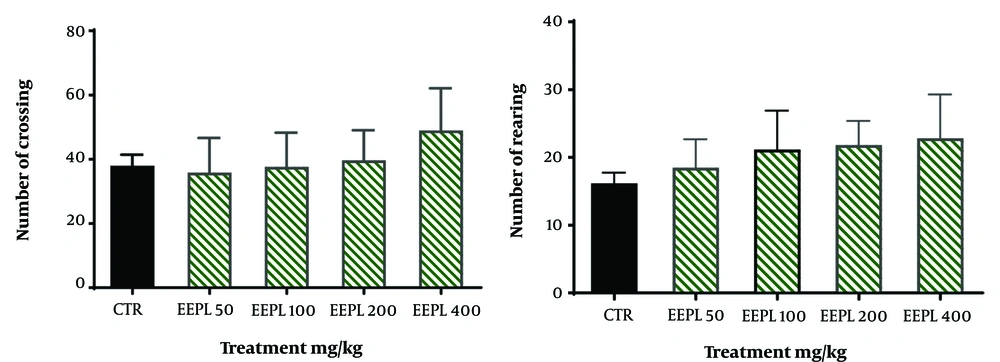
This test assesses the relationships between the antidepressant-like effect of the EEPL and animal locomotion (19). For this purpose, the mice were individually submitted to the center of the box, and the number of crossings and rearings was recorded during a 5-minute period.
3.6. The Treatment Protocols
3.6.1. Assessment of Ethanolic Extract of Pistacia lentiscus Antidepressant-like Activity in Tail Suspension Test and Open-Field Test
Different doses of EEPL (50, 100, 200, and 400 mg/kg), imipramine (as a tricyclic antidepressant, 30 mg/kg), and fluoxetine (as selective serotonin reuptake inhibitors, 20 mg /kg, i.p.) were administered 45 minutes before the behavioral tests. Control (vehicle) groups received normal saline plus DMSO (5%). In addition, to reduce the number of animals used, the open-field test (OFT) was performed 5 minutes before the TST.
3.6.2. Assessment of the Possible Mechanism of Action of Extract of Pistacia lentiscus in Tail Suspension Test
In order to evaluate the possible involvement of the dopaminergic system, the mice were pretreated with SCH23390 (a dopamine D1 receptor antagonist, 0.05 mg/kg), sulpiride (a dopamine D2 receptor antagonist, 50 mg/kg), or haloperidol (a non-selective dopamine receptor antagonist, 0.2 mg/kg). To evaluate the possible involvement of the serotonergic system, the mice were pretreated with WAY100135 (a selective 5-HT1A receptor antagonist, 10 mg/kg) and ketanserin (a selective 5HT2A/C receptor antagonist, 5 mg/kg). Moreover, the mice were pretreated with p-chlorophenylalanine (pCPA) (an inhibitor of serotonin synthesis, 150 mg/kg) or vehicle once a day for 3 consecutive days. Then, they received vehicle and EEPL (200 mg/kg) and were examined 30 min later.
To assess the noradrenergic system, the animals received yohimbine (an α2-adrenoreceptor antagonist, 1 mg/kg), prazosin (an α1-adrenoreceptor antagonist, 1 mg/kg), and propranolol (a β adrenoreceptor antagonist, 1 mg/kg) 1 hour before of the administration of vehicle and the extract. The animals were tested after 30 minutes. The doses of the EEPL and drugs used were selected on the basis of literature data and previous results from a study (19, 20).
3.7. Data Analysis
The collected data were expressed as mean ± standard deviation (SD). The data were analyzed using one (dose-response curves and time-course curves) or two-way (assessing the mechanism of action) analysis of variance (ANOVA) followed by Tukey’s post-hoc test in GraphPrism 9.0 software (Inc., San Diego, CA, USA). The P-value < 0.05 was considered a significant level. Six animals were considered in each group.
4. Results
4.1. Effects of Ethanolic Extract of Pistacia lentiscus on the Immobility Time in Tail Suspension Test and on the Animal Locomotion in Open-Field Test
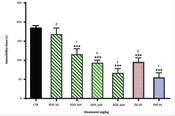
The one-way ANOVA shows that the acute administration of EEPL (100 - 400 mg/kg) could significantly reduce immobility time (37.5%, 49.3%, and 63.9% reduction, respectively) in a dose-dependent manner, compared to the control group (F6, 35 = 99.20, P < 0.001) in mouse TST (Figure 1). In addition, imipramine (30 mg/kg) (69.8% reduction) and fluoxetine (20 mg/kg) (48.2% reduction) as two standard drugs significantly decreased immobility time, compared to the control group (F6, 35 = 99.20, P < 0.001). In addition, a significant difference was not observed between the EEPL 400 mg/kg and imipramine group (P > 0.05). Moreover, there were no significant differences between the fluoxetine group and EEPL 200 mg/kg (P > 0.05). Therefore, the effects of the different doses of EEPL are similar to standard drugs. With regard to the results of the present study, 200 mg/kg of the extract was selected for the mechanism(s) of action.
The antidepressant-like effect of the EEPL (50 - 400 mg/kg) and common antidepressants in the TST (mean ± SD; n = 6) [*** presents a significant difference from the control group at P < 0.001; nevertheless, words (a-d) show significant differences from the EEPL (50 mg/kg) group at P < 0.05].
In order to detect the association of the EEPL antidepressant-like effect in TST to its locomotor activity, the animals were subjected to OFT. As shown in Figure 2, these doses could not alter animal locomotion in OFT (P > 0.05).
4.2. The Involvement of the Dopaminergic System on the Effect of Extract
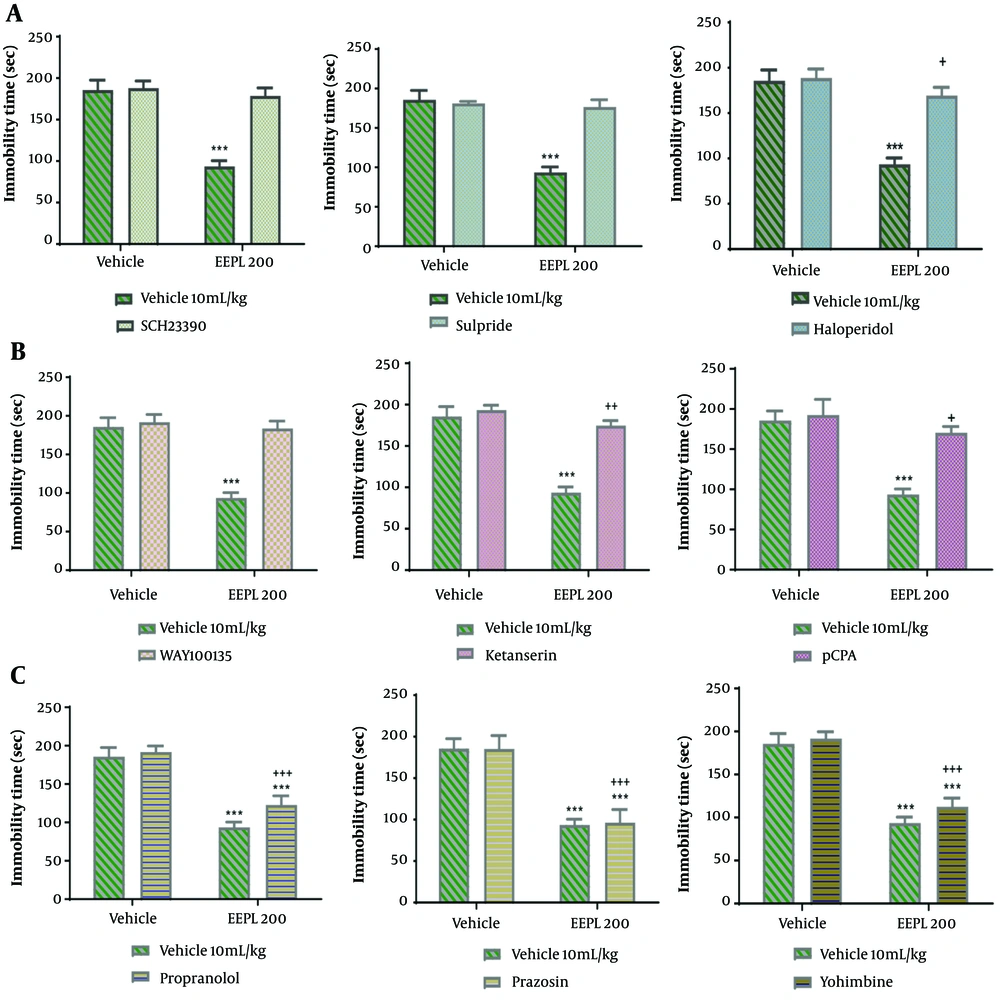
Figure 3A shows the effect of the pre-treatment of the animals with SCH23390 (a dopamine D1 receptor antagonist, 0.05 mg/kg), sulpiride (a dopamine D2 receptor antagonist, 50 mg/kg), or haloperidol (a non-selective dopamine receptor antagonist, 0.2 mg/kg) on the antidepressant potential of EEPL (200 mg/kg) in TST. The two-way ANOVA showed that the pre-treatment with SCH23390 [(P < 0.001); EEPL (P < 0.001); EEPL × SCH23390 (P < 0.001)], sulpiride [(P < 0.001); EEPL (P < 0.001); EEPL× sulpiride (P < 0.001)]and haloperidol [(P < 0.001); EEPL (P < 0.001); EEPL × haloperidol (P < 0.001)], prevented the interaction inhibited the antidepressant-like effect of the extract (200 mg/kg).
4.3. The Involvement of the Serotonergic System in the Effect of the Extract
Figure 3B shows the effect of the pre-treatment of the animals with WAY100135 (a selective 5-HT1A receptor antagonist, 10 mg/kg), ketanserin (a selective 5HT2A/C receptor antagonist, 5 mg/kg), and pCPA (an inhibitor of serotonin synthesis, 150 mg/kg) on the antidepressant potential of EEPL (200 mg/kg) in TST. The two-way ANOVA revealed that the pre-treatment with WAY100135 [(P < 0.001); EEPL (P < 0.001); EEPL × WAY100135 (P < 0.001)], ketanserin [(P < 0.001); EEPL (P < 0.001); EEPL× ketanserin (P < 0.001)]and pCPA [(P < 0.001); EEPL (P < 0.001); EEPL × pCPA (P < 0.001)], prevented the antidepressant-like effect of the extract (200mg/kg)
4.4. The Involvement of the Noradrenergic System in the Effect of the Extract
Figure 3C shows the effect of the pre-treatment of the animals with yohimbine (an α2-adrenoreceptor antagonist, 1 mg/kg), prazosin (an α1-adrenoreceptor antagonist, 1 mg/kg), and propranolol (a β adrenoreceptor antagonist, 1 mg/kg) on the antidepressant potential of EEPL (200 mg/kg) in TST. The two-way ANOVA showed that the pre-treatment of mice with yohimbine [(P = 0.004); EEPL (P < 0.001); EEPL × yohimbine (P = 0.108)], prazosin [(P = 0.811); EEPL (P < 0.001); EEPL× prazosin (P = 0.770)] and propranolol [(P = 0.004); EEPL (P < 0.001); EEPL × propranolol (P = 0.108)] could not prevent the antidepressant-like effect of the extract.
The effects of pretreating mice with different dopaminergic, serotonergic, and noradrenergic receptor antagonists on the antidepressant-like effect of EEPL (200 mg/kg) in TST (mean ± SD; n = 6) (*** shows a significant difference from the vehicle groups at P < 0.001; +, ++, +++ shows a significant difference from the EEPL group at P < 0.05, P < 0.01, and P < 0.001, respectively).
5. Discussion
This study evaluated the antidepressant-like effect of the EEPL in TST as a mouse model. The EEPL showed antidepressant-like effects in doses of 100 - 400 mg/kg. It could compete with conventional agents (e.g., fluoxetine and imipramine) in the highest doses. These findings are in line with previous studies reporting the reduction of immobility time by antidepressant agents (10, 15, 19, 21). Moreover, the OFT was conducted to determine false positive responses; however, no psychostimulant effect was observed. It showed that the decrease in immobility time in TST cannot be associated with the psychostimulant effects of EEPL. The results of the present study are in accordance with literature demonstrating that the antidepressants could not affect animal locomotion in the OFT (10, 15, 19, 21)
No studies could be found evaluating the antidepressant activity of EEPL. However, the effects of EEPL in the treatment of memory dysfunction have been reported (22).
Oxidative stress and pro-inflammatory signaling have significant roles in the pathogenesis of major depression (23). In this regard, previous studies have shown the anti-inflammatory activity of EEPL (11, 20). Moreover, the nervous system’s tissues are the most sensitive to oxidative stress (20). Antioxidant (24-26) and antidepressant-like activity (10, 14, 19, 27, 28) of EEPL could be attributed to its compounds, such as phenolic compounds (e.g., phenolic acids, flavonoids, tannins, stilbenes, and lignans).
In the current study, the monoaminergic system was evaluated as a possible mechanism of EEPL in TST. In this study, the pre-treatment of the mice with SCH23390 (a dopamine D1 receptor antagonist), sulpiride (a dopamine D2 receptor antagonist), or haloperidol (a non-selective dopamine receptor antagonist) significantly blocked the antidepressant-like effect of the EEPL (200 mg/kg) in TST. Hence, the results of the present study illustrated that the dopaminergic system appears to be involved in the antidepressant-like effect of EEPL.
Studies have shown that depression has a close relationship with default in the dopaminergic system (5, 29). It has been reported that antidepressants that modulate the dopaminergic system are desirable options for the treatment of depression (21, 27).
Conventional antidepressants (e.g., imipramine and fluoxetine) also work through the dopaminergic system (19). The mechanism by which EEPL shows its effects on the dopaminergic system is not clear. However, it might exhibit its effects on the dopaminergic system by increasing dopamine levels in the brain, as reported by other studies (30, 31). On the other hand, the extract inhibits the oxidation of dopamine during depression and keeps normal levels in the brain owing to its antioxidant properties.
Apart from the above-mentioned findings, most of the P. lentiscus biological or pharmacological activities are related to the phenolic composition of this plant (10, 14, 24-28). In this regard, and parallel to the findings of the current study, earlier studies show that phenolic acids (e.g., gallic acid and rosmarinic acid) partly induced their antidepressant-like activity via the modulation of the dopaminergic system. In support of this view, the pretreatment of animals with dopaminergic (D1 and D2) receptor antagonists abolished the antidepressant-like effect of phenolic acids (32, 33).
The results showed that EEPL also showed its effects on the serotonergic system. The pretreatment of mice with WAY100135 (a selective 5-HT1A receptor antagonist), ketanserin (a selective 5HT2A/C receptor antagonist), and pCPA (an inhibitor of serotonin synthesis) prevented the antidepressant-like effects of (200 mg/kg). Hence, our results presented here demonstrate that the serotonergic system appears to be implicated in the antidepressant-like effect of EEPL. The modulation of the serotonergic system in depression has been reported (6). The efficiency of EEPL in decreasing depression could be attributed to its antioxidant activity that inhibits the oxidation of serotonin and its reuptake (19). Moreover, studies showed that some phenolic compounds induced their antidepressant-like through the participation of the serotonergic system. In this regard, the pretreatment of animals with serotonergic (5-HT2A/2C and 5-HT3) receptor antagonists blocked the antidepressant potential of phenolic compounds (e.g., gallic acid and ferulic acid) (32, 34).
The adrenergic system is also modulating in the depression. The results showed the administration of yohimbine (a α2-adrenoreceptor antagonist), prazosin (a α1-adrenoreceptor antagonist), and propranolol (a β adrenoreceptor antagonist) could not reverse the antidepressant-like effect of EEPL (200 mg/kg), which confirms the non-involvement of noradrenergic system in the antidepressant-like effect of EEPL. The findings of previous studies have supported the results of the present study and demonstrated that different kinds of phenolic acids might also produce different effects on the brain’s neurotransmitter systems (32-35). On the other hand, only some phenolic acids (e.g., ferulic acid and caffeic acid) induced their antidepressant-like activity via the modulation of the noradrenergic system. However, EEPL could not show its effects through this mechanism. Seemingly, EEPL exhibits its effects on depression through the modulation of dopaminergic and serotonergic systems.
However, the present study had certain limitations. Firstly, since this study demonstrated the antidepressant potential of EEPL in TST, it is necessary to investigate other mouse models of depression, such as FST, social defeat stressors, and chronic mild stress models. Secondly, an assessment of the effects of the main compounds (e.g., phenolic acids) of EEPL on depression is required. Thirdly, it is crucial to investigate the effects of other mechanisms that could be involved in EEPL’s antidepressant-like activity, such as the role of antioxidants and other neurotransmitter systems. Finally, the current study is a murine model and cannot be used for clinical purposes in humans until future clinical studies.
5.1. Conclusions
In conclusion, the results confirmed the possible efficiency of EEPL in the treatment of depression by dopaminergic and serotonergic mechanisms but not the noradrenergic system. Overall, the results suggested that EEPL exerted an antidepressant-like activity in the mouse model of depression, which might provide insights into the potential of EEPL in therapeutic implications for depression.
![The antidepressant-like effect of the EEPL (50 - 400 mg/kg) and common antidepressants in the TST (mean ± SD; n = 6) [*** presents a significant difference from the control group at P < 0.001; nevertheless, words (a-d) show significant differences from the EEPL (50 mg/kg) group at P < 0.05]. The antidepressant-like effect of the EEPL (50 - 400 mg/kg) and common antidepressants in the TST (mean ± SD; n = 6) [*** presents a significant difference from the control group at P < 0.001; nevertheless, words (a-d) show significant differences from the EEPL (50 mg/kg) group at P < 0.05].](https://services.brieflands.com/cdn/serve/3170b/1c31ae58244b483cada61352a5cc186efdc23a74/ans-140852-i001-F1-preview.webp)