Dear Readers,
The article titled "Investigating the Protective Effect of Ellagic Acid on Cholemic Nephropathy in Cholestatic Rats" (1), published in the Archives of Neuroscience (link or DOI), is being retracted at the request of the publisher's ethics committee, including the editor-in-chief.
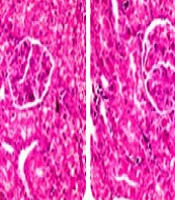
Following a report received from a reader on April 27, 2024 (ticket: #527727), regarding suspected plagiarism involving two main figures (Figures 4 and 5) in the results section of the mentioned article and concerns about the originality of the content, an investigation was conducted involving both the authors and reviewers.
The decision to retract this article was based on clear evidence of plagiarism (unattributed use of large portions of data, presented as figures) and concerns regarding the originality of the content. Specifically, it was found that Figures 4 and 5 in the published article bore a significant resemblance to figures from previously published works in the PharmaNutrition journal (2, 3), constituting a copyright violation:
- Figure 4 was copied from Figure 6 in the article "Hepatoprotective effect of boldine in a bile duct ligated rat model of cholestasis/cirrhosis" (2).
- Figure 5 was copied from Figure 8 in the article "Agmatine Alleviates Hepatic and Renal Injury in a Rat Model of Obstructive Jaundice" (3).
These findings indicate a breach of ethical publishing standards concerning the originality of the data presented. Despite requests for clarification from the authors, a sufficient explanation was not provided within the stipulated time frame.
In accordance with the guidelines provided by the Committee on Publication Ethics (COPE), the decision has been made to retract the article to maintain the integrity of the scientific record [COPE Guidelines on Plagiarism](https://publicationethics.org/resources/flowcharts/plagiarism-published-article).
We apologize to the readers of the journal for any inconvenience caused. The journal remains committed to upholding the highest standards of publication ethics and addressing any issues that arise in a timely and transparent manner.
Sincerely,
Publisher's Ethics Committee (Including the Editor-In-Chief)