1. Background
Intracranial meningiomas, the most common primary brain tumors, frequently present challenges in neurosurgery due to their vascular nature (1-3). These tumors receive blood from the dura and the adjacent pial vasculature (4-10). Surgical resection and the removal of associated dura and bone, if needed, remains the primary treatment for meningiomas. However, their hypervascularization can make resection difficult and may necessitate blood transfusion (8, 11).
In recent decades, preoperative embolization of meningiomas has been proposed to reduce intraoperative blood loss, facilitate safer resection, and decrease complications (12, 13). Yet, the benefits of preoperative embolization for intracranial meningiomas still need to be fully understood, and the procedure carries potential risks, particularly for large or giant meningiomas. These risks include intratumoral hemorrhage, ischemic stroke, post-embolization edema, and cranial nerve palsy (14-22).
While there is no precise definition of giant meningiomas, they are typically classified as those larger than 5 cm in diameter (23-25). These rare tumors present unique challenges for neurosurgeons due to their size, deeper location, and vascular supply. Giant meningiomas are also associated with longer surgery times, increased blood loss, and difficulties in achieving tumor exposure due to surrounding arteries and bridging veins (21, 22).
The authors propose that preoperative angiography and embolization of giant meningiomas could provide crucial information about the tumor's vascular supply, potentially leading to reduced intraoperative blood loss and shorter surgery times.
2. Objectives
This retrospective study aims to evaluate the safety and efficacy of preoperative embolization in patients with giant intracranial meningioma treated at a single center from January 2012 to December 2022.
3. Methods
This is a retrospective cohort study of patients diagnosed with cerebral meningioma, a subset of whom underwent preoperative meningioma embolization at our institution between January 2012 and December 2022. The decision to treat patients with preoperative angiography and possible tumor embolization was made by the treating neurosurgeons and neuroradiologists based on preoperative contrast-enhanced MRI. Reasons for considering preoperative tumor embolization included tumor size with a maximum diameter of more than 5 centimeters and tumor location. Medical records were reviewed, and demographic, clinical, and operative variables were collected, including age at treatment, sex, initial clinical symptoms, location of the tumor on preprocedural contrast-enhanced MRI, tumor size, location of the vascular supply, number of feeding pedicles, number of embolized feeders, embolization agents, and WHO tumor grade. Outcome variables included the degree of devascularization, intraprocedural complications, intraoperative complications, intraoperative blood loss, surgical time, postoperative complications, and whether blood transfusions were necessary.
Embolization procedures were performed with patients under either general endotracheal anesthesia or local anesthesia, depending on the patient's compliance and the date of surgery. The neurosurgeon and neuroradiologist's personal preference largely determined the embolization choice. Still, it was also correlated with high tumor vascularity, large tumor size, and challenging intraoperative access to arterial tumor supply. Angiographic imaging was conducted using high-resolution biplane subtraction angiography (biplane Allura Xper FD20/20, Philips Healthcare, Best, The Netherlands). Femoral artery access was usually obtained via a 6-French sheath. Standard diagnostic angiography was performed to analyze vascular anatomy and characterize the vascular supply of the meningioma. If embolization appeared feasible, selective catheterization of tumor-supplying vessels was conducted via a 6-French guiding catheter. Superselective catheterization of the feeding arteries was performed with a microcatheter, and vascular supply to healthy brain tissue or dangerous collaterals were excluded via selective angiography. Embolization was performed using 45 - 150 µm Contour polyvinyl alcohol (PVA) particles (Boston Scientific, Natick, Massachusetts, USA) and bare platinum coils and Onyx, depending on vessel anatomy, the presence of dangerous anastomoses and whether the patient was awake or under general anesthesia. After meningioma embolization, patients were brought either into the operating room for tumor resection or to the intensive care unit (ICU) for monitoring when tumor resection was scheduled for the next day.
The Statistical Package for Social Sciences-25 (SPSSv.25) was used to code variables of interest and perform statistical analyses. Descriptive statistics were used to calculate the mean and standard deviation (SD) of the different variables and the number of patients per category and associated percentages. The Pearson χ² test and Fisher exact test were performed to evaluate the differences between the treatment groups. Differences were considered significant for probability values of P < 0.05.
4. Results
A total of 189 patients with meningiomas were admitted to our institution between January 2012 and December 2022 for resection surgery. In 22 patients (11.6%), preoperative tumor embolization was performed. In nine embolized patients (40.1%), surgery was carried out on the same day, and these patients underwent both procedures under one general anesthesia.
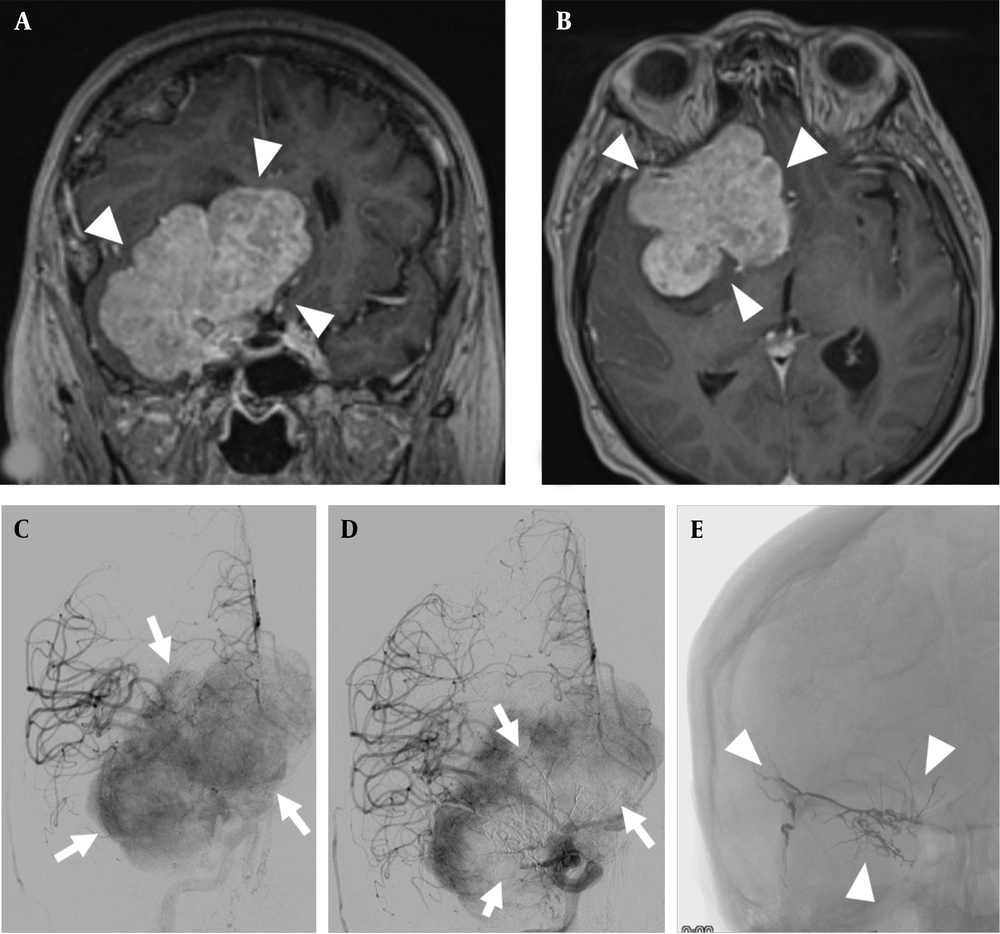
In 59.9% of the patients (n = 9), tumor resection was scheduled for the next day. Tumor embolization was possible under local anesthesia in seven of these nine patients. Figure 1 shows an example of a giant meningioma on the right greater sphenoid wing with pre- and post-embolization tumor angiography.
A and B, giant meningioma of the right greater sphenoid wing (arrowheads), coronal and transverse contrast-enhanced T1-weighted images; C, DSA with injection into the right common carotid artery showing a significant tumor blush in the late arterial phase (arrows); D, DSA with injection again into the right common carotid artery after embolization with a significant reduction in tumor blush in the center of the meningioma (arrows); E, radiograph without contrast injection showing the liquid embolic agent in the center of the tumor (arrowheads).
4.1. Baseline Characteristics
Table 1 summarizes the patient and tumor characteristics of all patients. When comparing age, sex, and WHO grade, no statistical differences were found between the treatment groups. The tumor volume was significantly more significant in the combined treatment group, with 59.2 cm3, compared to the surgery group with 15.7 cm3 (P = 0.002). Furthermore, tumor location and initial symptoms differed significantly between the groups (P < 0.001 and P = 0.004, respectively). In the combined treatment group, the main symptoms in 22.7% of the patients were cognitive deficits, and 18.1% had hemiparesis. In this group, only 13.6% showed no symptoms at presentation.
In contrast, in the surgery group, 37.7% were symptomless, and 13.8% underwent radiological diagnostics in the workup for a persistent headache, while 10.8% had epileptic seizures. Regarding tumor location, the most significant quantity in the combined treatment group presented with a sphenoid wing meningioma at 36.4%. In the surgery group, almost half of the patients were admitted with a convexity meningioma.
| Variables | Embolization + Surgery (N = 22) | Surgery (N = 167) | P-Value |
|---|---|---|---|
| Age (y) | 61.9 ± 12.0 | 61.1 ± 13.0 | 0.843 |
| Sex (female) | 12 (54.5) | 122 (73.1) | 0.084 |
| Initial symptoms | 0.004 | ||
| No symptoms | 3 (13.6) | 63 (37.7) | |
| Cognitive deficits | 5 (22.7) | 7 (4.2) | |
| Headache | 2 (9.1) | 23 (13.8) | |
| Visual deficits | 3 (13.6) | 9 (5.4) | |
| Hemiparesis | 4 (18.1) | 11 (6.6) | |
| Aphasia | 2 (9.1) | 10 (6.0) | |
| Dizziness | 1 (4.5) | 10 (6.0) | |
| Epileptic seizure | 1 (4.5) | 18 (10.8) | |
| Dysosmia | 1 (4.5) | 3 (1.8) | |
| Tumor location | <0.001 | ||
| Olfactory groove | 4 (18.1) | 7 (4.2) | |
| Sphenoid wing | 8 (36.4) | 11 (6.6) | |
| Parasagittal | 4 (18.1) | 17 (10.2) | |
| Falcine | 3 (13.6) | 34 (20.4) | |
| Tentorial | 1 (4.5) | 14 (8.4) | |
| Intraventricular | 1 (4.5) | 2 (1.2) | |
| Convexity | 1 (4.5) | 82 (49.1) | |
| Tumor size (cm3) | 59.2 ± 23.7 | 15.7 ± 20.8 | 0.002 |
| WHO grade | 0.789 | ||
| I | 19 (86.4) | 138 (82.6) | |
| II | 3 (13.6) | 26 (15.6) | |
| III | 0 (0) | 3 (1.8) |
4.2. Angiographic and Procedural Characteristics
Table 2 presents the angiographic and procedural characteristics of the combined treatment group. The mean number of feeding pedicles was 2.1, and the mean number of embolized pedicles was 1.5. In most patients, MMA embolization was performed at 81.8%, and particle agents were used for embolization at 90.9%. In 57.1% of the cases, an estimated devascularization of more than 75% was achieved.
| Variables | Embolization + Surgery (N = 22) |
|---|---|
| Number of feeding pedicles | 2.1 ± 0.8 |
| 1 | 4 (18.1) |
| 2 | 12 (55.5) |
| 3 | 3 (13.6) |
| 4 | 2 (9.1) |
| Number of embolized pedicles | 1.5 ± 0.6 |
| 1 | 13 (59.1) |
| 2 | 8 (36.4) |
| 3 | 1 (4.5) |
| Location of embolized pedicles | |
| Middle meningeal | 18 (81.8) |
| Ophthalmic | 5 (22.7) |
| Internal maxillary | 2 (9.1) |
| Anterior cerebral | 1 (4.5) |
| Temporal superficial | 2 (9.1) |
| Posterior cerebral | 2 (9.1) |
| Embolisates | |
| PVA particles | 20 (90.9) |
| Coils | 6 (27.2) |
| Onyx | 1 (4.5) |
| N-butyl cyanoacrylate | 3 (13.6) |
| Degree of devascularization | |
| ≥ 75 | 12 (57.1) |
| 50 - 74% | 9 (42.9) |
a Values are expressed as No (%) or mean ± SD.
4.3. Surgery Characteristics and Postoperative Complications
Table 3 presents the surgery characteristics and postoperative complications of the combined treatment group and the surgery group. In the combined treatment group, operation time and intraoperative blood loss were significantly higher than the surgery group (P = 0.023 and P < 0.001, respectively). The mean surgical time in the combined treatment group was 358.0 minutes, while it was 191.7 minutes in the surgery group. Additionally, the mean intraoperative blood loss was 581.8 milliliters in the combined treatment group, as opposed to 376.0 milliliters in the surgery group. Moreover, the 2 treatment groups had significant differences in postoperative complications (P = 0.014). In the combined treatment group, 1 patient experienced malignant edema, progressive stroke, and epileptic seizures, respectively, whereas secondary hemorrhage (2.4%) and epileptic seizures (4.8%) were most common in the surgery group. Notably, one rescue surgery was necessary in the combined treatment group due to malignant edema, whereas 4 rescue surgeries were required in the surgery group because of secondary hemorrhages (P = 0.465). It is worth mentioning that no intraprocedural complications occurred during angiography and embolization, and no intraoperative complications emerged during tumor resection in either group. Furthermore, no blood transfusion was required in the combined treatment group postoperatively, while 1 blood transfusion was administered postoperatively in the surgery group.
| Variables | Embolization + Surgery (N = 22) | Surgery (N = 167) | P-Value |
|---|---|---|---|
| Surgical time (min) | 358.0 ± 115.9 | 191.7 ± 93.0 | 0.023 |
| Intraoperative blood loss (mL) | 581.8 ± 195.5 | 376.0 ± 160.2 | <0.001 |
| Postoperative complications | 3 (13.6) | 16 (9.6) | 0.023 |
| Malignant edema | 1 (4.5) | 0 | |
| Secondary hemorrhage | 0 | 4 (2.4) | |
| Progressive stroke | 1 (4.5) | 0 | |
| Epileptic seizures | 1 (4.5) | 8 (4.8) | |
| Abscess/ wound infection | 0 | 2 (1.2) | |
| Aphasia | 0 | 1 (0.6) | |
| Pneumonia | 0 | 1 (0.6) | |
| Rescue surgery | 1 (4.5) | 4 (2.4) | 0.465 |
a Values are expressed as No (%) or mean ± SD.
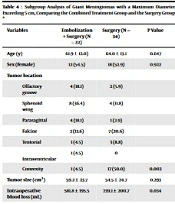
4.4. Subgroup Analysis of Giant Meningiomas
While there is no precise definition of giant meningiomas, most publications consider meningiomas larger than 5 cm in diameter as giant meningiomas (38 - 40). Table 4 presents the subgroup analysis of giant meningiomas, comparing the combined treatment and surgery groups. It is important to note that all patients who underwent preoperative meningioma embolization had meningiomas with a diameter exceeding 5 cm. Baseline characteristics, such as age and sex, exhibited no significant differences between the groups (P = 0.647 and P = 0.922, respectively). In terms of tumor location, a significant difference was observed (P = 0.003). In the surgery group, 50% of the tumors were in the convexity and 20.6% in the falcine region.
In contrast, in the combined treatment group, 36.4% of the tumors were situated in the sphenoid wing, while 18.1% were in the olfactory groove and parasagittal. Intraoperative blood loss was significantly lower in the combined treatment group, with a mean blood loss of 581.8 mL in preoperatively embolized patients compared to 739.1 mL in non-embolized patients (P = 0.034). However, surgical time did not exhibit a significant difference between the two groups (P = 0.570).
| Variables | Embolization + Surgery (N = 22) | Surgery (N = 34) | P-Value |
|---|---|---|---|
| Age (y) | 61.9 ± 12.0 | 64.0 ± 13.1 | 0.647 |
| Sex (female) | 12 (54.5) | 18 (52.9) | 0.922 |
| Tumor location | 0.003 | ||
| Olfactory groove | 4 (18.1) | 2 (5.9) | |
| Sphenoid wing | 8 (36.4) | 4 (11.8) | |
| Parasagittal | 4 (18.1) | 1 (2.9) | |
| Falcine | 3 (13.6) | 7 (20.6) | |
| Tentorial | 1 (4.5) | 3 (8.8) | |
| Intraventricular | 1 (4.5) | 0 | |
| Convexity | 1 (4.5) | 17 (50.0) | |
| Tumor size (cm3) | 59.2 ± 23.7 | 54.5 ± 24.7 | 0.293 |
| Intraoperative blood loss (mL) | 581.8 ± 195.5 | 739.1 ± 200.7 | 0.034 |
| Surgical time (min) | 358.0 ± 115.9 | 275.7 ± 110.1 | 0.570 |
| Rescue surgery | 1 (4.5) | 0 | 0.210 |
a Values are expressed as No (%) or mean ± SD.
5. Discussion
The current literature shows that the potential benefits of preoperative meningioma embolization, including reduced intraoperative blood loss, operation time, and tumor softening, have been widely reported (10, 20, 26-29). On the contrary, the risks involve ischemic complications, hemorrhages, cranial nerve deficits, and peritumoral edema (30-32). However, it remains unclear whether preoperative embolization improves the surgical outcome of meningioma patients. One potential reason may be the appropriate patient selection. The patients we selected for preoperative embolization differed significantly in tumor size and tumor location from those who underwent surgery alone. The largest group of embolized tumors in our study were skull base meningiomas. Raper et al. illustrated that meningiomas targeted for preoperative embolization tend to be larger and in deeper locations compared to those not referred to embolization (20), which is consistent with our patient cohort. They also showed that the vascular supply of skull base meningiomas is frequently difficult to access in the early stages of resection. Therefore, resection of these tumors was associated with higher blood loss and a lower chance of gross resection compared to convexity lesions (20). Consequently, skull base meningiomas are an attractive target for preoperative embolization. However, it is well known that the vascular supply of these tumors is variable and complex, with important anastomotic connections between the external and internal carotid arteries and vital neurological structures (33). These anastomotic connections can also be found in the vascular network of the meningioma itself (33, 34). Thus, aggressive embolization may lead to permanent post-procedural neurological deficits. Rosen et al. reported that 21.6% of the patients showed post-procedural complications, including nine percent with significant neurological deficits after 24 hours (35). The current literature reports that most complications occur during or within a few hours of procedural completion (19). The overall complication rates after meningioma embolization vary between 6 and 21% in the literature (11, 19, 35).
In our study, major neurological complications were found in 2 embolized patients postoperatively, 9.1%. One patient with an embolized convexity meningioma showed no neurological symptoms immediately after embolization and tumor resection the following day and could subsequently be discharged 14 days later. However, 22 days after resection, the patient was re-admitted with a middle cerebral artery stroke. In this patient, only embolization of the middle meningeal artery (MMA) was carried out; therefore, we think this complication is unrelated to the embolization procedure. The second patient underwent embolization of a sphenoid wing meningioma and tumor resection the subsequent day. Two days after resection surgery, the patient showed reduced vigilance, and the CT scan indicated progressive edema with midline shift. Rescue surgery in the form of decompressive hemicraniectomy was necessary. After rescue surgery and neurological rehabilitation, the patient had no permanent neurological deficits at follow-up. In this case, it remains unclear whether the embolization procedure, resection surgery, or a combination of both caused the malignant edema.
Several studies have suggested a beneficial effect of preoperative meningioma embolization. Some retrospective cohort studies have referred to reduced blood loss, a reduced need for transfusion, and fewer complications in embolized patients with no significant neurological deficits or adverse long-term effects (29, 36). However, Bendszus et al. found that significantly reduced blood loss may only be achieved by complete tumor devascularization in a prospective comparative cohort study (27). Furthermore, Waldron et al. reported a prospective case series with good outcomes after preoperative embolization of skull base meningiomas fed by the internal carotid artery, with a low complication rate (11).
Moreover, the adequate time interval between meningioma embolization and tumor resection remains controversial, with no preference for either early or delayed tumor resection after tumor embolization. Kai et al. suggested that one week after embolization, tumor resection leads to greater tumor softening and eases the resection, reducing blood loss and edema (23, 37). Another study predicted that after preoperative embolization with Onyx, surgery should be delayed for more than 10 days due to less edema (37). In contrast, shorter intervals between one and 7 days were recommended based on possible tumor revascularization and subsequent collateralization. In all of these studies, nonabsorbable embolization agents have been advised (21, 24, 25, 32).
At our institution, resection surgery was scheduled either directly after meningioma embolization or within 24 hours due to a possible increase in peritumoral edema post-procedurally, leading to elevated intracranial pressure. Based on the early tumor resection after embolization, tumors may not reach the ideal softening, thus limiting the improvement of surgical outcomes such as blood loss and operation time. However, our subgroup analysis of giant meningiomas with a more than 5 centimeters diameter showed significantly reduced intraoperative blood loss in preoperatively embolized patients. Additionally, operation time did not differ significantly between the groups, although the proportion of patients with superficial meningiomas was significantly lower in the combined group.
This study has several limitations, such as the retrospective review of medical records, which may contain incomplete or missing data, and the self-assessment of complications. The selection of patients to undergo preoperative meningioma embolization was based on individual surgeon preference, which may have resulted in considerable bias. In addition, as a single-center study, the study population is small. After correcting for tumor size, our study showed significantly reduced intraoperative blood loss in embolized compared to non-embolized patients with giant meningiomas. Although the 2 subgroups differed significantly in tumor location, with more tumors in deeper areas in the group of patients with embolization, there was no significant difference in surgical time. In our patients, the intra- and post-procedural complication rate was low compared with the existing literature. This likely depends on the availability of endovascular therapy options and may vary from site to site. However, further standardized prospective randomized controlled trials are needed to draw compelling conclusions about the role of preoperative embolization, with one focus on patients with giant meningiomas.
5.1. Conclusions
In our study cohort, preoperative embolization in appropriately selected patients with giant intracranial meningiomas was safe and feasible and showed a substantial degree of tumor devascularization with an acceptable low rate of complications. This might have led to a positive effect on intraoperative blood loss and duration of surgery.