1. Background
Schizophrenia is a complex and chronic disorder with a recurrent nature that can place a significant burden on families and society (1). The causes of schizophrenia spectrum disorders are believed to be multifactorial, with both genetic and environmental factors, as well as gene-environment interactions, playing a role in the course of this disorder (2). The immune function is also influenced by these interactions (3). There is evidence to support the important role of both adaptive and innate immune responses in the pathogenesis of schizophrenia and related psychotic disorders by affecting neurodevelopment, neurotransmitters, and neurodegeneration (4).
Studies have indicated a link between autoimmune diseases and psychotic disorders (5), and there is evidence suggesting the mediating effects of inflammatory pathways on environmental risk factors (6). These findings have gained increased attention to the role of inflammation in the pathogenesis of psychotic disorders (5, 6). Likewise, the serum concentrations of inflammatory cytokines, which are the key components of the immune system, may be altered in patients with psychosis several years before the onset of the disease (7). A meta-analysis of several cross-sectional studies revealed that inflammation may play a role in schizophrenia. Cytokines, such as interleukin-6 (IL-6), interleukin-1β (IL-1β), and transforming growth factor-β (TGF-β), seem to be present during the acute phase of the disorder and may serve as a state marker for schizophrenia (8).
Studies have investigated IL-6, which has both pro-inflammatory and anti-inflammatory effects, for its potential use as a biomarker for schizophrenia. The role of IL-6 in the development, progression, symptoms, and treatment of schizophrenia has been also extensively studied (9). Some evidence suggests significantly higher serum levels of IL-6 and its soluble receptor (sIL-6R) in patients with schizophrenia (10, 11). Another study reported higher IL-6 and lower IL-17 levels in patients with schizophrenia versus healthy controls (12). Meanwhile, there are some contradictory studies indicating no changes in the serum level of IL-6 (13, 14).
It is known that IL-12 is primarily involved in the development of autoimmune diseases, such as autoimmune encephalomyelitis and multiple sclerosis (15-18). Nonetheless, in patients with schizophrenia, there has been limited research on the serum concentration of IL-12, and the results have been inconsistent (19-21). Studies have shown that serum levels of IL-12 are higher in drug-naïve patients experiencing first-episode psychosis compared to healthy controls, with an increase observed after six weeks of receiving antipsychotic medications (19). Moreover, the mean level of IL-12β subunit, p40, is significantly higher in patients with schizophrenia (22), although some studies have reported no alterations (22).
In line with the abovementioned findings, the activity of immune cells may be more accurately represented by analyzing the patterns of gene expression in peripheral blood mononuclear cells (PBMCs). The gene expression patterns of stress mediators and cytokines in PBMCs are similar to those observed in the brain (23). Research evaluating the expression of these genes rather than circulating cytokines alone may help overcome some of the methodological challenges that make it difficult to draw conclusions about the role of the immune system in schizophrenia.
While few studies have measured both circulating cytokines and their expression levels in fresh blood cells, the results have been inconsistent. Some studies have reported higher levels of these cytokines in patients with psychosis (14, 24, 25), while others have observed lower levels of IL-6 gene expression in the PBMCs of these patients (26). It is worth mentioning that some of these studies did not recruit a comparison group of healthy controls, making it difficult to draw definitive conclusions (27). In light of these clinical and experimental findings, in this study, we aimed to measure the serum levels of IL-6 and IL-12, as well as the expression levels of IL-6 and IL-12 genes in an Iranian Azeri Turkish population. There is currently limited information available about patients with psychotic disorders from the Middle East, including Iran, and only a few studies have investigated genetic risk factors for these disorders in this population (28).
2. Objectives
We aimed to determine whether serum levels of IL-6 and IL-12 are altered in drug-naïve patients with recent-onset non-affective psychosis. We also attempted to determine if there are any differences in the mRNA expression of IL-6 and IL-12 between these patients and healthy controls and to explore any potential relationship between the serum or gene expression levels of these cytokines and clinical symptoms.
3. Methods
The present study was carried out within the framework of the Azeri Recent-Onset Acute Phase Psychosis Survey (ARAS) (29) in 2020 in Tabriz, Iran. The study protocol was approved by the regional ethics committee (No: IR.TBZMED.REC.1397.832). All participants and/or their caregivers signed written informed consent forms.
3.1. Participants
Adult patients with a recent episode (< 2 years) of non-affective psychosis were enrolled in this study. The study included patients with a diagnosis of schizophrenia, delusional disorder, schizophreniform disorder, brief psychotic disorder, schizotypal personality disorder, or schizoaffective disorder on the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV) (30). The exclusion criteria were as follows: diagnosis of a psychotic disorder caused by a general medical condition; diagnosis of affective psychosis; substance-induced psychotic disorder; any type of allergy; autoimmune disorders; signs and symptoms of an infection; and use of immunosuppressive or anti-inflammatory medications. The Positive and Negative Syndrome Scale (PANSS) was utilized to rate the severity of symptoms. The Positive and Negative Syndrome Scale (PANSS) is commonly used in the assessment of schizophrenia. The PANSS is a standardized tool that evaluates positive and negative symptoms, as well as general psychopathology associated with schizophrenia. It helps clinicians assess the severity of symptoms and track changes over time in individuals with schizophrenia (31).
A healthy control group was selected through local announcements in health centers and universities. Individuals aged 18 - 45 years, without any previous or current psychiatric disorder—based on the Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-I) (32)—were included in the study. Meanwhile, people with a recorded history of psychiatric disorders in their first-degree relatives were excluded. Similarly, patients with the current use of immunosuppressive or anti-inflammatory medications, any type of substance abuse (e.g., cigarettes), any type of allergy, autoimmune disorders, or signs and symptoms of infection were eliminated. The participants’ demographic data were recorded, and the healthy control group was matched to the patient group by age and gender.
3.2. Procedure
Sample collection and processing: A peripheral blood sample was collected from each participant using sterile venipuncture and placed into a tube containing 0.5 M EDTA anticoagulant. Using the Ficoll-Paque process (GE Healthcare Life Sciences, USA), peripheral blood mononuclear cells (PBMCs) were collected. Subsequently, the cream-colored inter-layer was rinsed with the Hank’s Balanced Salt Solution (HBSS #14170-161, Gibco™, USA) to remove any remaining platelets, serum, or other contaminants. The PBMC samples were pelleted at 2,000 rpm for 10 minutes at 10°C.
RNA Extraction and cDNA Synthesis: To isolate total RNA, TRIzol reagent (Life Technologies, USA) was used in accordance with the manufacturer's guidelines. The extracted RNA was assessed using agarose gel electrophoresis and a NanoDrop 2000C spectrophotometer (Thermo-Fisher Scientific, USA). Next, 3 µg of extracted RNA was treated with DNase I (#EN0521, Thermo-Fisher Scientific, USA) and used for cDNA synthesis. A Takara cDNA Reverse Transcription Kit (Takara, USA) was used for the cDNA synthesis. The Takara SYBR Green Master Mix (RR820L) was also used to measure the expression levels.
PCA and Gene Expression Analysis: A polymerase chain reaction (PCR) was performed in a LightCycler® 96 real-time PCR system (Roche Life Science, Switzerland) under the following conditions: 95°C for three minutes, followed by 45 cycles of 95°C for 10 seconds, 59°C for 30 seconds, and 72°C for 20 seconds. The cycle threshold (CT) values were determined for the relative quantification of gene expression. All values were standardized to the expression of the GAPDH housekeeping gene (9, 33, 34). The primer sequences are described in Table 1.
| Primers | Left | Right |
|---|---|---|
| IL-6 | GAGGAGACTTGCCTGGTGAAA | CAGCTCTGGCTTGTTCCTCAC |
| IL-12 | CTCCTCCTTGTGGCTACCCTG | GCCTTCTGGAGCATGTTGCTG |
| GAPDH | CGAGATCCCTCCAAAATCAA | TTCACACCCATGACGAACAT |
Serum Cytokine Assessment: In parallel, the serum levels of Il-6 and IL-12 were assessed using a sandwich ELISA kit (Bioassay Technology La, USA).
3.3. Statistical Analysis
For all statistical analyses in this study, GraphPad Prism Version 6.0 was used. First, the Shapiro-Wilk test was performed to investigate whether the expression levels of IL-6 and IL-12 mRNA had a normal distribution in both groups. Data are presented as mean ± standard error (SE) or standard deviation (SD). Additionally, to assess the normality of data, the Kolmogorov-Smirnov test was conducted. Unpaired t-tests were also used to examine differences between the groups. A P-value < 0.05 was considered statistically significant. Moreover, the diagnostic value of variables (as binary classifiers) that were significantly different between the two groups was evaluated based on the receiver operating characteristic (ROC) curve. Receiver operating characteristic analysis is used to quantify how accurately medical diagnostic tests (or systems) can discriminate between two patient states, typically referred to as "diseased" and "non-diseased." An ROC curve is based on the notion of a "separator" scale, on which results in the diseased and nondiseased form a pair of overlapping distributions. The complete separation of the two underlying distributions implies a perfectly discriminating test, while complete overlap implies no discrimination. The ROC curve shows the tradeoff between the true positive fraction (TPF) and false positive fraction (FPF) as one changes the criterion for positivity (35). The area under the ROC curve (AUC) reflects the ability of the target variable to reliably discriminate between patients and healthy controls. An AUC value of 0.50 suggests that the target variable has no diagnostic value, while an AUC value >0.50 reveals that the target variable has a greater probability of discriminating between patients and healthy controls in a randomly selected sample.
4. Results
In this study, we examined 40 drug-naïve patients and 40 healthy controls, all of whom self-reported having an Azeri Turkish background. The participants’ demographic characteristics are presented in Table 2. Only five patients were non-smokers in this study. The mean body mass index (BMI) of the participants was 23.9 ± 3.1 kg/m2.
| Variables | Patients (n = 40) | Healthy Controls (n = 40) | P-Value |
|---|---|---|---|
| Male/Female | 2.07 | 1.35 | 0.3560 |
| Mean age | 29.78 (9.60) | 30.33 (8.58) | 0.7880 |
| Married | 9 (22.5) | 18 (45.0) | 0.009 |
| Occupation | 14 (35.0) | 31 (77.5) | < 0.0001 |
| Mean years of education | 9.1 (3.2) | 15.6 (2.5) | 0.0451 |
| Score of positive symptoms | 16.73 (4.33) | -- | -- |
| Score of negative symptoms | 10.05 (3.35) | -- | -- |
| Global Assessment of functioning (GAF) score | 30.0 (6.4) | 91.1 (5.1) | 0.0011 |
| IL-12 mRNA level (*10-3) | 2.508 ± 4.911 | 1.99 ± 2.965 | 0.5697 |
| IL-6 mRNA level (*10-2) | 34.36 ± 17.86 | 5.437 ± 6.733 | < 0.0001 |
| Serum level of IL-12 (pg/mL) | 0.77 (0.85) | 1.29 (1.36) | 0.0777 |
| Serum level of IL-6 (pg/mL) | 42.75 (24.64) | 7.01 (9.32) | < 0.0005 |
a Values are presented as No. (%) or mean ± SD.
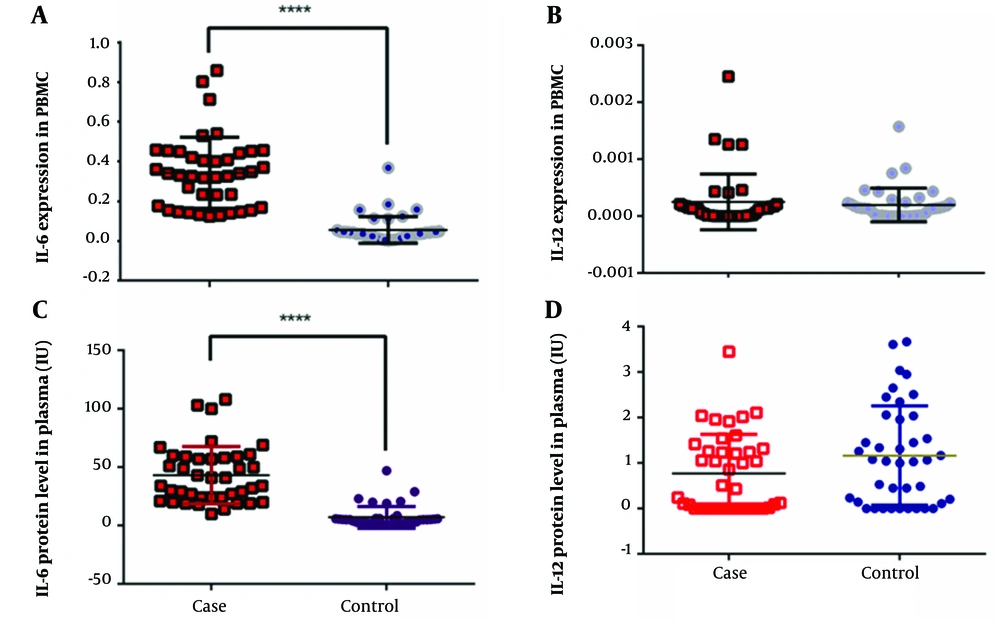
The normal distribution of data was approved for the IL-6 and IL-12 mRNA levels in the two groups. An unpaired t-test and F-test were performed to analyze the gene expression of IL-6 and IL-12. Based on the results, the patients had a significantly higher level of IL-6 mRNA gene expression in comparison to the normal controls (t = 9.58, F = 7.037, P < 0.0001); the corresponding values are presented in Table 2 and Figure 1. However, this difference was not significant for IL-12 (t = 0.5709, F = 2.743, P = 0.5697). Similar findings were observed for the serum levels of IL-6 and IL-12. According to Figure 1, the serum level of IL-6 was significantly higher in the patient group (t = 8.738, F = 6.889, P < 0.0001), whereas the serum level of IL-12 was not significantly different between the two groups (t = 1.788, F = 1.634, P = 0.0777).
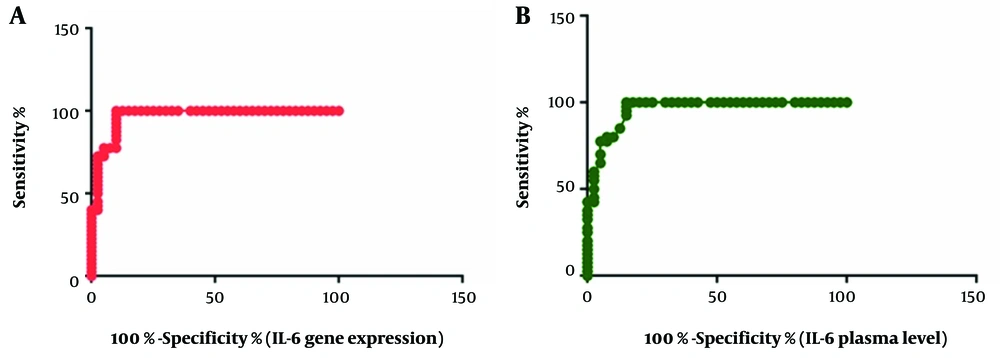
Next, the diagnostic value of IL-6 mRNA level and its serum concentration was examined based on the ROC curve. Noticeably, AUC was 0.9669 for the IL-6 mRNA level (95% CI: 0.9299-1.004, P < 0.0001; Figure 2) and 0.9584 for the serum concentration of IL-6 (95% CI: 0.9184-0.9985, P < 0.0001; Figure 2). A serum IL-6 level of 9.320 pg/mL was the best cutoff point for diagnosis, with a sensitivity of 100% and a specificity of 85%. Also, a CT value of 0.1239 was estimated as the best cutoff point for IL-6 mRNA expression, with a sensitivity and specificity of 100% and 90%, respectively.
The AUC values for IL-12 gene expression and its serum concentration were measured to be 0.5413 (95% CI: 0.4132-0.6693, P = 0.5254) and 0.6066 (95% CI: 0.4826-0.7305, P = 0.1009), respectively. The correlation between mRNA gene expression and the serum level of both proteins was also examined. The results showed that higher levels of PBMC mRNA expression were associated with higher serum concentrations of proteins (IL-6: R = 0.9705, P < 0.0001; IL-12: R = 0.1788, P < 0.0001). Based on the findings, the serum concentration of IL-6 was not correlated with the serum level of IL-12 (P = 0.815). The same pattern was observed for the level of gene expression (P = 0.507). Finally, it was found that the levels of IL-6 and IL-12 were not correlated with the severity of symptoms, gender, or age of the patients.
5. Discussion
This study compared IL-6 and IL-12 gene expression and serum levels in freshly collected PBMC samples between Iranian Azeri patients with first-episode psychosis and a healthy control group matched by gender and age. According to the results, the mRNA expression and serum protein levels of IL-6, but not IL-12, were significantly higher in patients with schizophrenia. The results also showed a correlation between IL-6 mRNA expression and its serum concentration. The AUCs for both IL-6 mRNA expression and serum concentration exceeded 0.9, demonstrating the excellent ability of these indices to differentiate between patients with first-episode psychosis and healthy individuals. According to this finding, the level of IL-6 could accurately distinguish between patients with recent-onset non-affective psychosis and healthy individuals in our sample. Despite the complexity of the immunological pathway, our findings suggest that IL-6 levels may be a useful biomarker for the diagnosis of schizophrenia.
As mentioned by Upthegrove et al., the relationship between pro-inflammatory conditions and the development of schizophrenia remains a longstanding issue in this field (11). The results of the present study, which demonstrated an increase in IL-6 levels, provide further support for the findings of research of some other authors like Upthegrove, Kushner, Stojanovic, and others (11, 36-43); nevertheless, they still contradict the results of few other studies like studies of Cazzullo, Borovcanin, and Garver (13-15, 44). It should be noted that there are some methodological differences between studies measuring the serum level of IL-6 (37, 42, 45, 46) in terms of sampling tissues (e.g., adipose, muscle, liver, or blood). Additionally, as mentioned by Ikeda et al. and Karstoft and Pedersen, this population is at an increased risk of metabolic dysregulation, which may influence the results (47, 48). On the other hand, interpreting the source, cause, and implications of the serum protein level can be difficult, as it originates from all tissues in the body. To partly address this limitation, we measured both the gene expression and serum level of IL-6 and IL-12 in PBMCs.
Previous studies, like the studies of Chase et al., have proposed that IL-6 may serve as a state marker for schizophrenia, with increased serum levels observed during both the first episode of psychosis and acute relapse (8, 9). These results are somewhat consistent with our observations, indicating that the underlying pathophysiology of schizophrenia may be associated with a high level of inflammation. In our study, no significant correlation was found between the level of IL-6 and the severity of symptoms in recent-onset schizophrenia; therefore, the level of IL-6 may be considered a state diagnostic marker in this population. Furthermore, research by Stojanovic et al. has shown that IL-6 levels are elevated in individuals at a high risk of schizophrenia, with the highest levels observed in those who have progressed to the development of acute psychosis (43); this finding highlights its potential as a diagnostic tool for early detection of schizophrenia. It is also important to replicate and compare these findings with other psychiatric disorders, especially depression.
Meanwhile, our study found no significant differences in IL-12 mRNA expression or serum protein levels between patients with psychosis and healthy controls. This finding is contrary to the results of research by Muller et al. Schwarz et al. and contrary to our initial assumption that higher levels of IL-12 indicate an inflammatory process (49, 50). While the mean levels of IL-12 were not significantly different between the two groups, a significant difference was observed in the distribution of corresponding values, as reflected by the high SD values (0.85 vs. 1.36). Our findings suggest a significant degree of heterogeneity among patients with psychosis, as demonstrated by the varying levels of IL-12. Accordingly, while some patients may have normal IL-12 levels, a subgroup of patients have substantially elevated IL-12 levels during the afflicted episodes, suggesting that immune system disturbances may be only present in a specific group of patients. An earlier study supports the notion of heterogeneity among patients with psychosis, as it found that individuals with treatment-resistant schizophrenia had significantly higher serum IL-6 levels compared to healthy controls, while other patients did not show any significant differences in the serum IL-6 levels (40).
Interestingly, the study of Crespo-Facorro et al. found that IL-12 levels were high in patients with schizophrenia and increased after six weeks of antipsychotic therapy (19). Another study (Kim et al.) found that the levels of IL-12 and TGF-β1 significantly increased in the patient group compared to those who were drug naïve and had not yet received any treatment. At week eight of treatment, the level of TGF-β1 returned to control values, whereas IL-12 levels remained relatively unchanged (51). In our study, the level of IL-12 in drug-naïve patients was not significantly higher than that of the control group, which contradicts previous reports. However, further studies on patients who are in different stages of the disorder are necessary to draw a definitive conclusion.
Some previous studies, like studies of Stojanovic et al. and Luo et al., have proposed a correlation between the circulatory level of IL-6 and the severity of symptoms in individuals with first-episode psychosis (43, 52), which was not replicated in our study. Generally, the level of cytokines may be influenced by several factors, including the use of medications. It should be noted that patients in this study were recruited during their first episode of psychosis and were either drug naïve or had recently (within a maximum of two weeks) started taking medications that were unlikely to affect cytokine levels.
5.1. Limitations
This study had some limitations. First, the blood sampling procedure could partly influence the results. In this study, blood samples were stored in deep freezers for up to three months until mRNA extraction. However, a recent study showed that storage for up to three years did not affect the indicators of the inflammatory response system (53). Second, there are several technical challenges that may influence the results of studies in comparable ways. For instance, the process of thawing samples can lead to an increase in IL-6 and other cytokines (54). Also, multiple confounding factors, including BMI, age, gender, smoking habits, current or recent infectious diseases, and use of medications, have been suggested to influence the serum level of cytokines (55). It should be noted that our study groups were matched in terms of age and gender, and those with a recent infectious disease or medication use were excluded. However, unlike our patients, the healthy controls were non-smokers, and their BMI data were not available. Although we found that the levels of cytokines and mRNA expression were not significantly correlated with the BMI of the patients, this should be addressed in further studies.
5.2. Conclusions
This study provided further evidence supporting the significant role of inflammation in the development of psychosis. The results showed that drug-naïve patients with first-episode non-affective psychosis had elevated concentrations of IL-6 but not IL-12 compared to healthy individuals. Therefore, circulating IL-6 levels may discriminate patients with first-episode psychosis from healthy controls. Our study found no discrepancy between the gene expression and serum levels of these proteins, and they had no correlation with the clinical symptoms.