1. Background
One of the most prevalent anxiety disorders in the general population and medical settings is generalized anxiety disorder (GAD) (1, 2). GAD manifestations including constant fears and worries about dangers for family members’ life, job, economic status, and health problems should last for at least 6 months to make the diagnosis. In addition, there are some physical symptoms related to GAD that can improve the diagnosis such as dizziness, paresthesia, flashes, tension in muscles, sensation of external object in the throat, having pain in the chest, not have concentration, dryness in the mouth, augmented heart rate, gastrointestinal as well as respiratory disturbances, and having problem sleeping (1). Currently, the prevalence of GAD has been estimated to be 1.6% to 5.0% in the general population and 2.8% to 8.5% in medical settings (2, 3). Based on the report of a recent study in Kashan, Iran from 2008 to 2009, GAD was the most prevalent subset of anxiety disturbances with a prevalence rate of 7.2% among psychotic patients (4). The exact etiology of GAD is not fully understood. However, studies pointed to genetic, psychological, and neurobiological factors. Among the neurobiological factors, the disorders in GABAergic neurotransmitters can be mentioned (5, 6). Although serotonin-norepinephrine reuptake inhibitors (SNRIs), selective serotonin reuptake inhibitors (SSRIs), and pregabalin are first-line therapies for GAD, about half of the patients did not show the predicted results (1, 6). Moreover, the side effects related to some anxiolytic drugs impose an adverse impact on treatment adherence. The unsatisfactory efficacy of pharmacological treatment, particularly when long term tolerability and remission rate were considered, encouraged non-pharmacological approaches as an adjuvant therapy (6).
Crocus sativus L. stigma, commonly known as saffron, is a perennial stemless herb of the Iridaceae family that is widely cultivated in Iran and other countries such as India and Greece (7, 8). In traditional medicine, saffron stigma is used for anxiety, depression, and insomnia (7, 9, 10). Five randomized clinical trials (RCT) reported that stigma and hydro-alcoholic extract of Crocus sativus can significantly attenuate symptoms of depression in patients with major depressive disorders (8, 11-16). In 3 of the mentioned clinical trials, short-term administration of hydro-alcoholic extract of Crocus sativus L appeared to be as efficient as fluoxetine (8, 13, 16) and imipramine (11). These outcomes became more interesting with regard to saffron’s notable tolerability and safety profile. It seems that 2 major components of saffron, safranal, and crocin block the reuptake of serotonin, norepinephrine, and dopamine (7, 14). As saffron has previously been approved as an effective therapy in depression and when compared to SSRIs (13, 14, 16), it might alleviate GAD symptoms as well. In the two only preclinical studies, saffron and saffron preparations showed anxiolytic effects and also increased the total sleep time (17, 18). Since up to the best of our knowledge no human studies have assessed the therapeutic effect of saffron as an adjuvant therapy in GAD patients, we designed the current study.
2. Methods
2.1. Study Participants
In the current randomized double-blind clinical trial, 40 GAD patients between the ages of 18 and 55 years who were referred to a private referral psychiatric clinic were recruited with stratify sampling. Patients were diagnosed with GAD according to diagnostic and statistical manual of mental disorders-V (DSM-V) criteria (19), based on Hamilton anxiety rating scale (HAM-A) scores of 18 - 24 (mild to moderate anxiety) (20). Exclusion criteria included pregnancy and lactation, receiving antipsychotic medications in a month prior to the recruitment, drug abuse, and suffering from other psychological disorders (e.g. bipolar disorder, schizophrenia, mood disorders; based on the diagnosis of our study psychologist or having a registered medical history or using specific medications). Written informed consent was obtained from all patients. The protocol for this project has been approved by our university and the trial was registered in clinicaltrials.gov (ID number: NCT02800733).
2.2. Intervention
At recruitment, weight, height, body mass index, general physical examination, 3day food recalls, and 14-items Hamilton anxiety questionnaire were recorded. This semi-structured interview was used to determine the patient’s anxiety level (20). Anxiety levels based on this scale ranged from 18 to 25 for mild to moderate and 25 to 30 for moderate to severe anxiety.
Furthermore, patients were randomly allocated to the saffron and placebo group using random blocks. Patients in the saffron group consumed a 500 mg-capsule containing 450 mg saffron on daily bases for 6 weeks in addition to sertraline (50 mg). Patients in the placebo group received a 500-mg capsule of starch with the same protocol. Patients were visited at the end of the 3rd as well as 6th week and 3day food recall and HAM-A total score were recorded. To exclude the effect of nutrients, which might affect GAD, 3days of food recall including 1 holyday were recorded at each visit. The daily intake of energy, carbohydrate, fiber, total fat, saturated fatty acids, monounsaturated fatty acids (MUFA), polyunsaturated fatty acids (PUFA), cholesterol, vitamin E, C, and B group, selenium, zinc, and iron were measured using food recalls using Nutritionist-4 Software. The adherence to treatment was measured by counting the returned unused capsules at the end of the 3rd and 6th week. Patients with less than 70% adherence were excluded. The patients were asked not to change their dietary habits and physical activity level. Patients were also asked to report any unexpected side effects to the psychiatrist of our team by phone call throughout the study. Changes in total HAM-A score was considered as treatment effects.
2.3. Statistical Methods
The sample size calculation was done considering α = 0.05, β = 0.2, power = 80%, and S = 5. Under these assumptions, at least 15 subjects in each group were determined to be necessary to demonstrate a significant difference between placebo and saffron groups. After checking for the normality of data using Kolmogorov-Smirnov test, independent sample t-test, and Mann-Whitney U test were applied to compare normally and non-normally distributed variables at each visit between saffron treating and placebo receiving groups, respectively. For categorical data, differences between groups was determined using Fisher’s Exact Test. Repeated measures test was used to assess within group changes between baseline, 3rd, and 6th week visit in each group. Analysis of covariance (ANCOVA) was used to assess the total HAM-A score differences between the 2 studied groups at each time point after adjusting for age, baseline energy intake as well as HAM-A total score, and weight changes from baseline to the 6th week. P-value less than 0.05 were considered as significant. All tests were 2-tailed. SPSS 21 (SPSS, Chicago, IL) was used to analyze the data.
3. Results
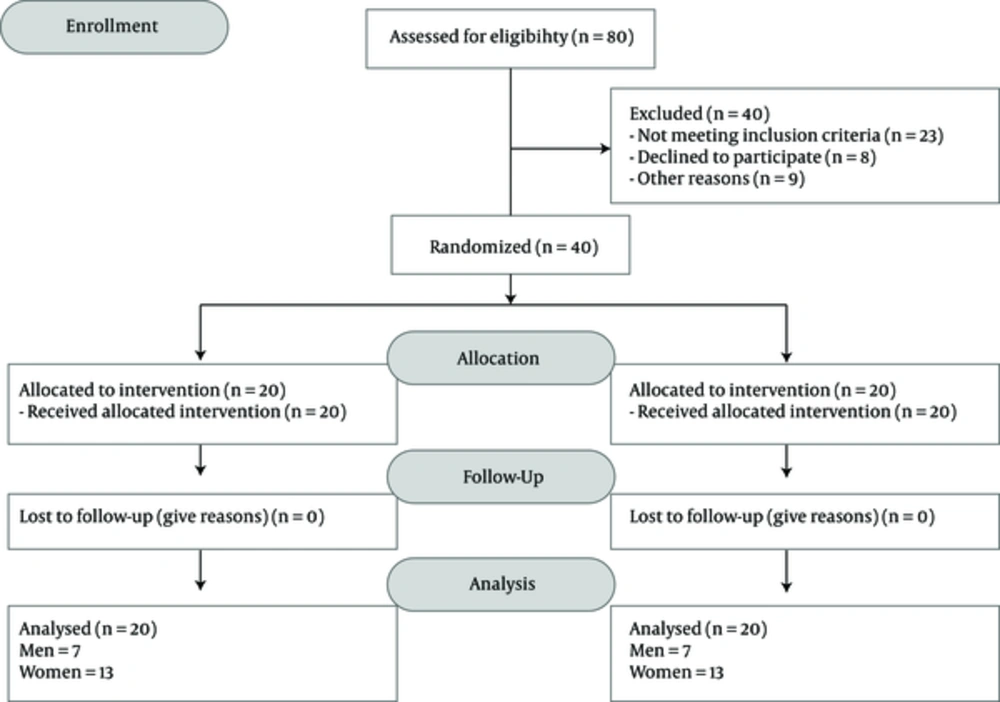
In each group, 13 women and 7 men were recruited. Figure 1 presents the flow diagram of the studied participants. The mean (SD) of age was 29.65 (8.45) and 32.40 (6.74) years in the Saffron and placebo groups, respectively (Table 1). At baseline, no significant differences were noted in age, gender, and marital status between the 2 studied groups (Table 1).
| Variable | Saffron Group (n = 20) | Placebo Group (n = 20) | P Value |
|---|---|---|---|
| Genderb | 1.000 | ||
| Female | 13 (65.0) | 13 (65.0) | |
| Marital Statusb | 0.748 | ||
| Single | 7 (35.0) | 8 (42.1) | |
| Married | 13 (65.0) | 11 (57.9) | |
| Agec | 29.65 (8.45) | 32.40 (6.74) | 0.262 |
| Total daily caloriec, kcal/d | 2838.02 (1210.96) | 2733.50 (570.27) | 0.730 |
| Total daily proteinc, g/d | 106.00 (68.00 - 402.00) | 106.50 (66.00 - 165.00) | 0.355 |
| The total daily carbohydratec, g/d | 244.30 (90.7 - 1505) | 207.25 ( 121.60 - 476.30) | 0.665 |
| Total daily fat, g/d | 61.00 (35.00 - 360.00) | 61.00 (34.00 - 102.00) | 0.253 |
| Total fiberc, g/d | 29.24 (14.59) | 26.24 (5.26) | 0.396 |
| Total daily cholesterolc, g/d | 431.60 (142.10) | 477.06 (105.98) | 0.259 |
| Saturated fatty acidsd, g/d | 18.41 (9.01 - 152.68) | 15.67 (9.47 - 32.69) | 0.265 |
| Fatty acids with a double bondd, g/d | 18.72 (9.03 - 154.79) | 15.39 (8.90 - 32.41) | 0.102 |
| Omega-3 fatty acidsd, g/d | 0.62 (0.18 - 27.45) | 0.46 (0.20 - 0.77) | 0.038 |
| Omega-6 fatty acids, g/d | 15.61 (3.75 - 58.40) | 9.98 (4.09 - 26.60) | 0.108 |
| Vitamin Ed, mg/d | 6.90 (4.56 - 29.09) | 6.97 (4.27 - 14.01) | 0.967 |
| Vitamin Cc, mg/d | 99.96 (35.88) | 87.56 (26.16) | 0.219 |
| Iron intaked, mg/d | 25.35 (12.00 - 125.40) | 23.58 (15.50 - 34.16) | 0.314 |
| Seleniumd, mg/d | 0.17 (0.10 - 2.01) | 0.16 (0.10 - 0.53) | 0.883 |
| Zincd, mg/d | 14.70 (8.02 - 276.46) | 13.98 (7.99 - 20.36) | 0.231 |
| Thiamin, mg/d‡ | 3.10 (1.39 - 26.75) | 3.04 (1.93 - 4.89) | 0.989 |
| Riboflavind, mg/d | 2.02 (1.29 - 27.08) | 1.82 (1.01 - 3.29) | 0.414 |
| Niacind, ‡mg/d | 27.47 (17.48 - 94.69) | 31.11 (16.93 - 47.39) | 0.862 |
| Vitamin B6d, mg/d | 2.09 (1.19 - 8.54) | 2.16 (1.32 - 3.68) | 0.820 |
| Folatec, µg/d | 685.97 (240.82) | 657.52 (191.91) | 0.682 |
| Cobalaminc, µg/d | 4.07 (2.18) | 3.18 (1.33) | 0.128 |
| Pantothenic acidc, mg/d | 7.01 (2.28) | 7.78 (2.34) | 0.296 |
| Biotinc, µg/d | 20.65 (7.62) | 24.10 (10.21) | 0.233 |
aValues are expressed as No. (%), mean (SD) or median (minimum-maximum) when applicable.
bBetween groups comparisons were made using Fisher’s Exact Test.
cIndependent samples T test.
dMann-Whitney U test.
Table 1 also shows the dietary intakes of the study participants. When comparing the 2 studied groups, baseline dietary intakes were similar, except for the estimated daily median intake of omega-3 fatty acids, which was significantly higher in the saffron group (0.62 g/day) compared to placebo group (0.46 g/day) (P value = 0.038 for between groups comparison; Table 1).
Table 2 presents the comparison of changes in weight and BMI during the study period. Comparing the 2 groups at each time point of the study does not show any significant differences in these anthropometric characteristics (Table 2). However, the results within group comparisons demonstrate a significant increase in the weight and BMI of the participants. Changes in mean weight and BMI, relative to baseline, were +1.09 kg and + 0.38 kg/m2 in the intervention group and +1.03 kg + 0.39 kg/m2 in the placebo receiving group, respectively (P value ≤ 0.000 for within group comparisons; Table 2).
| Variable | Group | Base Line | 3rd Week | 6th Week | Changes from Baseline to 6th Week | P Valuec |
|---|---|---|---|---|---|---|
| BMI | Saffron Group (n = 20) | 26.33 (5.12) a | 26.45 (5.14) a | 26.70 (5.04) a | + 0.38 (0.44) | 0.000 |
| Placebo Group (n = 20) | 25.49 (5.90) a | 25.61 (5.90) b | 25.88 (5.88) a,b | + 0.39 (0.52) | 0.000 | |
| P Valued | 0.634 | 0.635 | 0.637 | 0.918 | ||
| Weight | Saffron Group (n = 20) | 69.37 (12.45) a | 69.74 (12.64) a | 71.89e (13.71) a | +1.09 (1.21) a | 0.000 |
| Placebo Group (n = 20) | 71.30 (16.80) a | 71.63 (16.67) b | 72.33 (16.44) a,b | +1.03 (1.37) a | 0.000 | |
| P Valued | 0.687 | 0.694 | 0.929 | 0.865 |
aValues are expressed as mean (SD).
bAlphabets represent significant differences between each variable and two other variables, calculated by Bonferroni test (post-hoc).
cWithin group comparisons were made using Repeated Measure ANOVA.
dBetween groups comparisons were made using Independent samples T test.
eP value < 0.05 considered as significant.
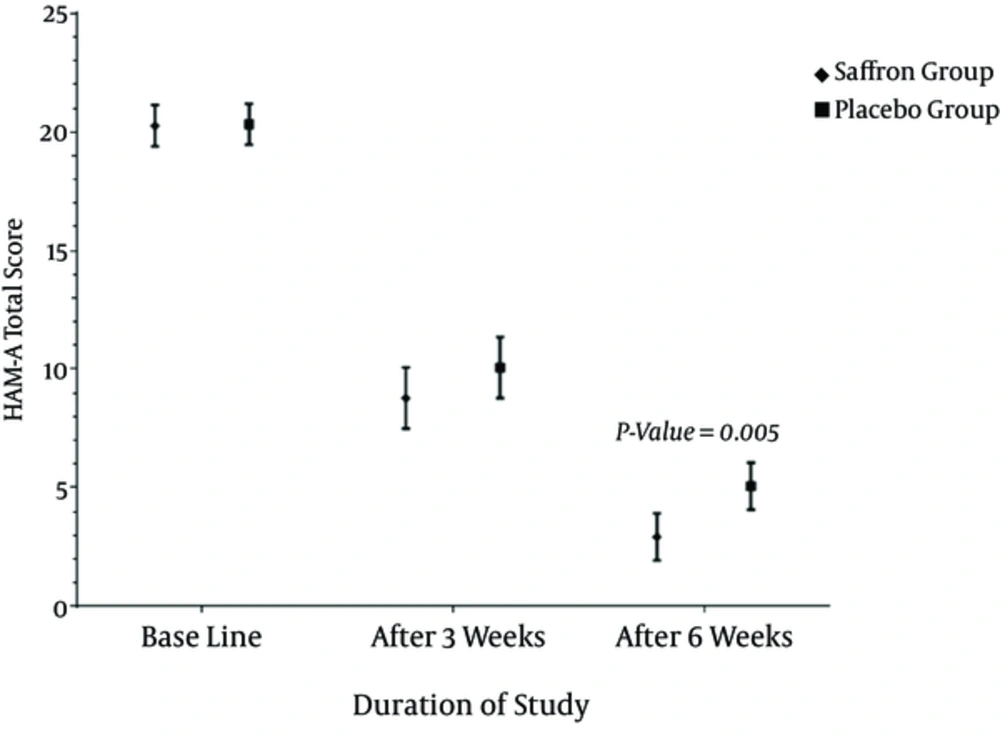
No significant difference in total HAM-A score was observed between saffron treated and placebo receiving groups at baseline (Table 3). The mean HAM-A score in saffron treated patients significantly declined from 20.20 at baseline to 8.65 at the end of the 3rd week and 2.95 at the end of 6th week. Furthermore, the mean HAM-A score of the placebo groups were 20.40, 10.20, and 5.05 at baseline and at the end of the 3rd and 6th week, respectively (P value ≤ 0.000 for within group comparisons; Table 3 and Figure 1). Applying ANCOVA models adjusted for age, baseline energy intake, and HAM-A total score, as well as weight changes from baseline to the 6th week, for the between group comparisons, showed that after three weeks of intervention there was no significant difference in HAM-A total score between the 2 studied groups. However, at the end of the 6th week saffron treated patients had a significantly lower mean HAM-A score compared to placebo group (2.95 vs. 5.05 P value = 0.005; Table 3 and Figure 2).
| Variable | Group | Base Line | 3rd Week | 6th Week | Mean (SD) Changes from Baseline to 6th Week | P Valuec |
|---|---|---|---|---|---|---|
| Total HAM-A Score | Saffron Group (n = 20) | 20.20 (19.20 - 21.19) a | 8.65 (7.34 - 9.95) a | 2.95 (1.98 - 3.91) a | -17.25 (2.67) | 0.000 |
| Placebo Group (n = 20) | 20.40 (19.74 - 21.05) a | 10.20 (8.92 - 11.47) a | 5.05 (3.92 - 6.17) a | -15.35 (2.30) | 0.000 | |
| P Value | 0.921d | 0.172e | 0.005d,f | 0.029d |
Abbreviations: DSM-V, diagnostic and statistical manual of mental disorders-V; GAD, generalized anxiety disorder; HAM-A, Hamilton anxiety scale.
aValues are expressed as unadjusted mean (95%CI).
bAlphabets represent significant differences between each variable and two other variables, calculated by Bonferroni test (post-hoc).
cWithin group comparisons were made using Repeated Measure ANOVA.
dBetween groups comparisons were made using ANCOVA models adjusted for age, energy intake at baseline, and weight changes from baseline to 6th week.
eBetween groups comparisons were made using ANCOVA models adjusted for age, baseline energy intake and HAM-A total score and weight changes from baseline to 6th week.
fP value < 0.05 considered as significant.
Measuring changes in the HAM-A total score relative to baseline and following adjustment of ANCOVA models for age, weight changes from baseline to 6th week, and energy intake at baseline, demonstrated that saffron was more effective than placebo in reducing the mean HAM-A total score of the study participants (-17.25 ± 2.67 vs. -15.35 ± 2.30, P value = 0.029; Table 3 and Figure 2).
Figure 2 provides the time related reduction in the mean HAM-A score of the 2 studied group at each time point after the intervention.
Regarding assessing the adverse effects of the intervention, 4 patients of the saffron group reported side effects includes constipation (1 patient), polydipsia (1 patient), and headache (2 patients). The side effects were tolerable and did not result in discontinuation of the supplementation.
4. Discussion
The results of the present study showed that 6 weeks of treatment with saffron as an adjuvant therapy to sertraline improved GAD significantly, which is in accordance with the results of the previously performed animal studies (17, 18). Experimental evidence indicated to the anxiolytic effect the crocin (17, 21). In a study carried out in mice, low doses of the aqueous extracts of saffron (56 and 80 mg/kg) and safranal (0.15 and 0.35 mL/kg) caused anxiolytic like effects, not different from that of diazepam (3 mg/kg), explained by increment in the time spent in the open arms of an elevated plus maze (18). Administration of aqueous extracts of saffron (1 - 10 mg/kg), and crocin (1 - 10 mg/kg) reduced stress-induced anorexia in the mice without influencing the plasma corticosterone levels (22). These results suggest an anti-stress effect of saffron and crocin. Furthermore,our results are in accordance with the results of studies, which compared saffron with SNRIs in mild to moderate depression (11, 13, 16). For example, in a 6-week randomized controlled trial study, the effect of 30 mg per day of saffron administration on 30 depressed patients was compared with 100 mg per day of imipramine. It was reported that saffron as an antidepressant agent can be as effective as imipramine (11). Additionally, comparing the antidepressant effect of 30 mg per day of hydro-alcoholic extract saffron with 20 mg per day of fluoxetine on 30 patients during a 6-week trial showed the same results (16). In addition, the administration of saffron petal 15 mg 2 times a day demonstrated the same antidepressant effect as fluoxetine 10 mg 2times a day after 8 weeks (13). The neurobiological factors include disturbances of various neurotransmitter systems (serotonin, epinephrine/ nor epinephrine, GABA) are thought to be potential etiological factors for GAD and other anxiety disorders (1, 23). Thus, the observed effect might be explained by the affinity of saffron components including crocetin and crocin to glutamate receptors, which resulted in increased glutamate neurotransmission and subsequent improvement of major depressive disorder signs (7, 11, 24). In a study on male Wistar rats, intraperitoneal injection of different doses of saffron aqueous extract (50, 100, 150, and 250 mg/kg) have been shown to increase brain dopamine level in a dose-dependent manner. The concentration of glutamate has also demonstrated to be increased after injection of the 250 mg/kg (highest dosage) of saffron aqueous extract (25).
The potential effects of saffron on improving a wide range of mental diseases have been confirmed. Owing to the crucial role of disturbed neurotransmitters such as glutamate, GABA, 5-HT and dopamine in the pathogenesis of anxiety disorders, the main constituents of saffron safranal and crocin can be effective in improving the disorders mainly through suppressing the reuptake of monoamines including norepinephrine, serotonin, and dopamine. The other main effects of this medicinal plant in enhancing mental disorders are as follows: GABAergic and serotoninergic effects, suppressing N-methyl-D-aspartate (NMDA), suppressing monoamine oxidase, modifying neural and endocrine system, as well as inhibiting the increase in corticosterone levels in plasma due to accelerated stress levels (24, 26-29).
It is interesting to note that there was a similar slight trend in increasing BMI and body weight in saffron treated and placebo taking groups in current study. A possible explanation for this might be that all studied patients were taking sertraline as their main anxiolytic drug. It has been reported that long term taking of the drug may result in slight weight gain as a side effect (24, 30).
The limitations of our study include a slightly short duration in addition to ethical constraints that was not possible to assess the effects of saffron alone -without sertraline or any other prescribed medication- on GAD. Furthermore, the study was limited to the patients who received sertraline. However, in order to exclude the effect of medication, we had to choose one drug for all patients.
4.1. Conclusions
Our data showed the beneficial effects of saffron as an add-on therapy to sertraline for GAD patients. However, more randomized clinical trials with larger sample size and longer duration of follow-up are needed to confirm this effect.