1. Background
Surgeries can be painful for patients, and reducing patient pain is a key medical strategy in surgery. Effective management of postoperative pain is a vital component of nasal surgeries because inadequate pain control can lead to various consequences, including decreased patient satisfaction, prolonged hospitalization, high doses of analgesics, and increased healthcare costs. In this regard, pain reduction techniques are used to alleviate patient discomfort, such as administering analgesics before, during, or immediately after surgery (1). Despite using various surgical techniques and different medications to reduce pain, there is no consensus on the optimal approach to pain management and avoiding excessive postoperative prescriptions in rhinoplasty and septoplasty surgeries (2, 3). The use of opioids for pain control after these types of nasal surgeries, which are typically considered outpatient procedures, also presents significant challenges. The availability of these medications in the market is limited, and even if they are obtained, prescribing them to patients is concerning due to potential side effects, such as respiratory depression. Consequently, the implementation of methods that reduce the need for opioid injections and analgesics is crucial for minimizing adverse effects and improving patient satisfaction (4, 5).
The sphenopalatine ganglion is one of the important neural structures located in the posterior part of the nasal area. Blocking this nerve has proven highly effective in reducing facial pain, and it is commonly used to treat various painful conditions through sphenopalatine ganglion nerve block (SPGB) (6). Studies indicate that SPGB in endoscopic sinus surgeries can reduce the need for narcotics in the postoperative period (7, 8). Moreover, this method has proven effective in successfully controlling bleeding and its related complications (1). The management of bleeding during the procedure is another crucial aspect of endoscopic nasal and sinus surgeries. Various measures have been implemented to minimize and control bleeding in both open and endoscopic nasal surgeries, and different techniques have been developed and utilized to improve the surgical site's quality. However, no highly effective method has been definitively established as the preferred choice for achieving this objective (9-13). Considering the significant role of the sphenopalatine ganglion in controlling intraoperative bleeding and pain management during and after surgery, further studies are essential, particularly due to the limited research conducted on its relevance to rhinoplasty surgeries (4, 5, 14). Additionally, it is important to note that rhinoplasty is a cosmetic procedure, and postsurgical patient satisfaction is crucial in attracting patient referrals and serving marketing purposes.
2. Objectives
This study aimed to investigate the impact of SPGB on intraoperative bleeding and pain levels during and after rhinoplasty and septoplasty surgeries. We assessed the effects of SPGB with a 0.5% bupivacaine irrigation compared to a placebo irrigation with normal saline. The findings of this research can provide surgeons with additional strategies to alleviate patient pain and minimize bleeding during or after surgery.
3. Methods
3.1 Study Design
This study was conducted as a double-blind, randomized clinical trial involving 30 eligible patients who were scheduled for elective rhinoplasty and septoplasty surgeries at hospitals of the Tehran University of Medical Sciences. After providing a clear description of the study protocol and the potential risks and advantages associated with participation, written informed consent was obtained from each patient. Following the acquisition of approval from the local Ethics Committee, the participants were randomly assigned to 2 groups: The SPGB group and the placebo group. All the patients were informed that they might either undergo an SPGB or not based on random assignment. From April to December 2022, this study included individuals between the ages of 18 and 50 years who underwent rhinoplasty and septoplasty. Participants were required to have an American Society of Anesthesiologists (ASA) class of I or II, indicating they were either in good health or had a mild systemic illness without any functional limitations. Patients with a history of conditions such as stroke, coronary artery disease, hypertension, deep vein thrombosis, pulmonary embolism, peripheral vascular disease, or inherited blood disorders, as well as those using antiplatelet or antihypertensive medications, were excluded from the study. In this double-blind, randomized study, both the patients and the researchers/physicians involved in the study were unaware of the assigned treatment groups. This means that neither the individuals receiving the treatments nor the individuals administering the treatments and collecting the data had any information about the treatment of each participant. This blinding helped minimize potential biases and ensure the objectivity of the results.
3.2. Randomisation
We used a blocked randomization method to assign 30 patients at a 1: 1 ratio to either the SPGB or the placebo group. When the patients were admitted, they were randomly assigned to one of 2 groups: The SPGB group, which received 0.5% bupivacaine, or the placebo group, which received normal saline. We performed the randomization using random number tables generated using an online random number generator (https://www.graphpad.com/quickcalcs/randomize1.cfm). The random number generator provided a sequence of random numbers that we used to assign patients to the different treatment groups.
3.3. Experimental Procedures
A total of 30 patients were randomly allocated to the SPGB and the placebo groups. In the SPGB group, a swab saturated with 4 mL of 0.5% bupivacaine was inserted into both nasal cavities, reaching the posterior end. In the placebo group, a swab saturated with 4 mL of normal saline was inserted into both nasal cavities until the end, similar to the SPGB group.
The anesthesia procedure was performed similarly in both groups as follows: After routine monitoring, including blood pressure, pulse oximetry, electrocardiogram (ECG), and end-tidal carbon dioxide (ETCO2) measurement, fentanyl at a dose of 1 μg/kg, lidocaine at a dose of 1.5 mg/kg, and midazolam at a dose of 0.2 mg/kg were administered. Two minutes after induction, anesthesia was induced using propofol at a dose of 2.5 mg/kg and atracurium at a dose of 0.5 mg/kg. Following tracheal intubation, anesthesia maintenance was achieved using a propofol infusion at a rate of 100 μg/kg per minute and remifentanil at a dose of 1 mcg/kg/min, with controlled ventilation to maintain normocapnia and using 50% oxygen. At the end of the surgery, muscle relaxation was reversed with neostigmine at a dose of 0.04 mg/kg and atropine at a dose of 0.02 mg/kg. Prior to anesthesia induction, all the patients received isotonic crystalloids at a rate of 3 ml/kg, and during the surgery, a maintenance fluid was administered based on the patient's weight, with blood loss compensated using a ratio of 3: 1 with Ringer's lactate solution up to the allowable blood loss limit. The surgery began after a delay of 10 minutes to ensure that the block had enough time to develop.
3.4. Experimental Outcomes
To measure the amount of bleeding, in addition to gas counting, their weight was measured before and after surgery. The increase in gas weight was converted to volume in milliliters and added to the other measured volumes. These volumes included the volume in the suction chamber and the volumes of normal saline used to rinse the surgical cavity, which were combined to calculate the quantitative amount of bleeding. The surgeon's satisfaction with the surgical site bleeding was recorded based on the Boezaart table. Additionally, the quality score of the surgical site in terms of bleeding was recorded based on the Boezaart scale, as well as the surgeon's satisfaction with bleeding control at 30, 60, and 90 minutes. Pain during the operation was measured based on the amount of anesthetic used. Postoperative pain was assessed by the surgeon or anesthesia resident by interviewing the patient about pain at 2, 4, 6, and 24 hours after surgery and recording the responses. The Visual Analog Scale (VAS) was used as a measurement of pain. Preoperative and postoperative information, including patient details (age, weight), medical history (certain diseases or medication usage), and preoperative tests, such as hematocrit, platelet count, international normalized ratio (INR), partial thromboplastin time (PTT), and prothrombin time (PT), were documented in a checklist.
3.5. Statistical Analysis
The statistical analysis was conducted in SPSS software (IBM Corp., Armonk, NY, USA), and a significance level of P < 0.05 was deemed indicative of significance. The between-group differences were evaluated using either a t-test or Mann-Whitney U test for quantitative variables, and a chi-square test was employed for qualitative variables.
4. Results
4.1. Demographic Characteristics and Clinical Variables of the Patients
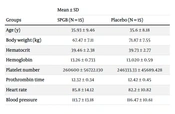
Thirty patients were allocated to either the SPGB group (n = 15) or the placebo group (n = 15). No significant statistical differences were observed between the 2 groups in terms of demographic and clinical characteristics, such as age (P > 0.05), body weight (P > 0.05), hematocrit (P > 0.05), hemoglobin (P > 0.05), platelet number (P > 0.05), prothrombin time (P > 0.05), heart rate (P > 0.05), and blood pressure (P > 0.05) (Table 1).
| Groups | Mean ± SD | |
|---|---|---|
| SPGB (N = 15) | Placebo (N = 15) | |
| Age (y) | 35.93 ± 9.46 | 35.6 ± 8.18 |
| Body weight (kg) | 67.47 ± 7.11 | 71.87 ± 7.55 |
| Hematocrit | 39.46 ± 2.38 | 39.73 ± 2.77 |
| Hemoglobin | 13.26 ± 0.733 | 13.020 ± 0.59 |
| Platelet number | 260600 ± 56722.130 | 246333.33 ± 45689.428 |
| Prothrombin time | 12.32 ± 0.34 | 12.42 ± 0.45 |
| Heart rate | 85.8 ± 14.12 | 82.2 ± 10.82 |
| Blood pressure | 113.7 ± 13.18 | 116.47 ± 10.61 |
Abbreviations: SD, standard deviation; SPGB, sphenopalatine ganglion nerve block.
In addition, certain variables that could potentially influence the results were evaluated. There were no significant differences between the 2 groups in ASA class (P > 0.05) and variables such as disease (P > 0.05), medication intake (P > 0.05), cigarette usage (P > 0.05), and acetaminophen use (P > 0.05) (Table 2).
| Groups | No. (%) | |
|---|---|---|
| SPGB (N = 15) | Placebo (N = 15) | |
| ASA | ||
| Class I | 13 (86.7) | 12 (80) |
| Class II | 2 (13.3) | 3 (20) |
| Disease | ||
| Anorexia | 1 (6.7) | 0 |
| Depression | 1 (6.7) | 0 |
| Headache | 0 | 1 (6.7) |
| Migraine | 1 (6.7) | 0 |
| Skin mole | 1 (6.7) | 0 |
| No disease | 11 (73.3) | 14 (93.3) |
| Medication intake | ||
| Nervous treatment | 1 (6.7) | 1 (6.7) |
| Chlordiazepoxide | 1 (6.7) | 1 (6.7) |
| Fluoxetine | 1 (6.7) | 0 |
| Sertraline | 1 (6.7) | 0 |
| No medication | 12 (80) | 13 (86.7) |
| Cigarette usage | ||
| Yes | 13 (86.7) | 13 (86.7) |
| No | 2 (13.3) | 2 (13.3) |
| Acetaminophen usage | ||
| Yes | 9 (60) | 7 (46.7) |
| No | 6 (40) | 8 (53.3) |
Abbreviations: ASA, American Society of Anesthesiologists; SPGB, sphenopalatine ganglion nerve block.
4.2. The Effect of Sphenopalatine Ganglion Block on Pain of Patients
Postoperative pain was assessed at multiple time points (2, 4, 6, and 24 hours) following surgery. The findings demonstrated a significant reduction in the VAS scores at each point in time within both groups (P = 0.028). This indicates that pain levels decreased significantly over time in both groups. However, when comparing the 2 groups, no significant differences were observed in the VAS scores (P = 0.166). These results suggest that while both groups experienced a reduction in pain, the treatment group did not exhibit a statistically significant advantage over the control group in terms of pain reduction (Table 3).
| Group | Mean ± SD | |||
|---|---|---|---|---|
| 2 hours | 4 hours | 6 hours | 24 hours | |
| SPGB | 0.53 ± 0.99 | 1.20 ± 1.37 | 0.93 ± 0.88 | 0.60 ± 0.50 |
| Placebo | 0.13 ± 0.35 | 0.47 ± 0.64 | 0.80 ± 0.56 | 0.60 ± 0.50 |
Abbreviation: SD, standard deviation; SPGB, sphenopalatine ganglion nerve block.
To evaluate intraoperative pain, we measured the total dose of fentanyl administered in both groups. The results revealed that the SPGB group had a significantly lower total fentanyl dose compared to the placebo group (P < 0.05). This significant difference in dosage indicates a pain reduction in the SPGB group. These findings provide strong evidence for the effectiveness of SPGB in pain reduction during intraoperative procedures. By achieving lower fentanyl requirements, SPGB demonstrates its potential as a viable approach for managing intraoperative pain and improving patient outcomes (Table 4).
| Group | Total Dose of Fentanyl (Mean ± SD) | |
|---|---|---|
| SPGB | 20 | 8.16 |
| Placebo | 76.67 | 9.59 |
Abbreviation: SD, standard deviation; SPGB, Sphenopalatine ganglion nerve block.
4.3. The Effect of Sphenopalatine Ganglion Block on Physician Satisfaction with Bleeding Amount
The amount of bleeding was measured at 30, 60, and 90-minute intervals during the operation. Our results showed that the SPGB group had significantly lower bleeding than the placebo group at 30 minutes (P < 0.05). However, there were no significant differences between the 2 groups at 60 and 90 minutes (P > 0.05) (Table 5).
| Groups Time and Bleeding | SPGB (N = 15) | Placebo (N = 15) | P-Value |
|---|---|---|---|
| 30 minutes | 0.035 | ||
| Without | 0 | 0 | |
| Mild | 10 | 3 | |
| Moderate | 4 | 9 | |
| Severe | 1 | 3 | |
| 60 minutes | 0.053 | ||
| Without | 1 | 1 | |
| Mild | 11 | 5 | |
| Moderate | 2 | 9 | |
| Severe | 1 | 0 | |
| 90 minutes | 0.35 | ||
| Without | 10 | 13 | |
| Mild | 4 | 2 | |
| Moderate | 1 | 0 | |
| Severe | 0 | 0 |
Abbreviation: SPGB, sphenopalatine ganglion nerve block.
4.4. The Effect of Sphenopalatine Ganglion Block on Bleeding According to Boezaart Criteria
According to the Boezaart criteria, bleeding in the SPGB group was significantly lower than in the placebo group at 30 minutes (P < 0.05) and 60 minutes (P < 0.05). However, no significant differences were observed between the 2 groups at 90 minutes (P > 0.05) (Table 6).
| Groups Time and Bleeding | SPGB (N = 15) | Placebo (N = 15) | P-Value |
|---|---|---|---|
| 30 minutes | 0.005 | ||
| Without | 0 | 0 | |
| Mild | 6 | 1 | |
| Mild with suctioning | 8 | 3 | |
| Low | 0 | 3 | |
| Moderate | 0 | 5 | |
| Severe | 1 | 3 | |
| 60 minutes | 0.016 | ||
| Without | 3 | 1 | |
| Mild | 7 | 1 | |
| Mild with suctioning | 4 | 6 | |
| Low | 0 | 5 | |
| Moderate | 0 | 2 | |
| Severe | 1 | 0 | |
| 90 minutes | 0.35 | ||
| Without | 10 | 12 | |
| Mild | 4 | 3 | |
| Mild with suctioning | 1 | 0 | |
| Low | 0 | 0 | |
| Moderate | 0 | 0 | |
| Severe | 0 | 0 |
Abbreviation: SPGB, sphenopalatine ganglion nerve block.
4.5. The Effect of Sphenopalatine Ganglion Block on Bleeding
Our results revealed that bleeding in the SPGB group was significantly lower than in the placebo group at 30 minutes (P = 0.00) (Table 7).
| Group | Bleeding (Mean ± SD) | |
|---|---|---|
| SPGB | 10.50 | 1.65 |
| Placebo | 17 | 1.36 |
Abbreviation: SD, standard deviation; SPGB, Sphenopalatine ganglion nerve block.
5. Discussion
The present study aimed to explore the effect of SPG nerve block using 0.5 bupivacaine injection on postoperative pain in rhinoplasty and septoplasty surgeries. This double-blind clinical trial was motivated by the costs associated with sedative consumption and the desire to minimize invasive methods for controlling vital signs. The results of this study revealed significant improvements in postoperative pain scores in both the placebo and SPGB groups. This suggests that both interventions independently contributed to pain reduction. However, it is noteworthy that no significant difference was observed between the 2 groups in terms of pain relief. Consistent with our results, Cho et al. demonstrated that the use of SPGB with bupivacaine did not significantly reduce postoperative pain after functional endoscopic sinus surgery (FESS) compared to the placebo (4). In another study, it has been reported that the use of SPGB with ropivacaine had a relatively reduction effect on reducing pain (5). Moreover, S. Cohen et al. reported that SPGB exhibited a remarkably positive effect on headaches (15). Several studies showed that the SPGB could significantly improve postdural puncture headaches (5, 15, 16). According to the study conducted by Ekici and Alagoz, the SPGB group revealed significant pain relief compared to the control group at 2, 6, 12, and 24 hours after surgery (17). In 2019, Rezaeian et al. assessed the impact of SPGB using bupivacaine on relieving postoperative pain in individuals who underwent endoscopic sinus surgery. They demonstrated that SPGB with bupivacaine 0.5% (1.5 mL) led to a significant reduction in postoperative pain at 24, 12, and 6 hours. However, there was no significant difference between the intervention and control groups at 48 hours, as well as on days 7 and 21 following the surgery (18). Another finding has indicated a significant reduction in postoperative pain within the first three hours after endonasal surgery in patients who underwent nerve block with 0.5 % bupivacaine compared to the control group. These findings highlight the impact of nerve blockage on postoperative pain in patients undergoing endonasal surgery (19).
In the present study, we observed a significant difference in the average amount of opioid (fentanyl) administered between the placebo and the SPGB groups. The placebo group received a higher average amount of fentanyl compared to the SPGB group. This finding indicates a significant difference between the 2 groups in terms of the quantity of fentanyl received. The difference in fentanyl administration suggests that the SPGB intervention may have contributed to reducing the need for opioids in postoperative pain management. This is an important finding, as opioids are associated with various side effects and risks, including respiratory depression, sedation, and nausea. By minimizing opioid consumption, the SPGB technique may offer potential benefits in terms of reducing the incidence of opioid-related complications and improving patient safety. The reduced requirement for fentanyl in the SPGB group may be attributed to the analgesic effects of the SPGB itself. The SPGB technique targets the sphenopalatine ganglion, which plays a crucial role in transmitting pain signals. By blocking this neural pathway, the SPGB may provide effective pain relief and reduce the need for additional analgesics, such as opioids. This finding aligns with previous research demonstrating the efficacy of SPGB in reducing analgesic requirements and improving postoperative pain control in various procedures. Gaafar et al. demonstrated that bilateral SPGB significantly improves the control of hemodynamics, intraoperative bleeding, average consumption of propofol and fentanyl during the procedure, and the requirement for postoperative analgesia in the blocked group compared to IV clonidine premedication (7). In consistence with our results, Gaafar et al. conducted a double-blinded and placebo-controlled study to assess the impact of regional blockade on opioid reception and recovery times following endoscopic sinus surgery compared to general anesthesia alone (7). They showed that SPGB significantly reduced the utilization of fentanyl during the recovery period compared to general anesthesia, resulting in a quicker hospital discharge for the patients (8). Degirmenci et al. investigated the effectiveness of transnasal SPBG in managing postoperative pain following septorhinoplasty. The findings indicated that transnasal SPBG provides a valuable approach for alleviating pain and reducing the requirement for additional pain-relieving medications within the first 24 hours following the procedure (20). In another study, they demonstrated a significant reduction in the average dosage of paracetamol and tramadol utilized within the initial 24 hours after the procedure in the block group. Additionally, the number of patients requiring analgesics was lower in the block group compared to the control group (20).
In our study, we conducted a comparison of the Boezaart scale to assess bleeding incidence and severity between the SPGB and placebo groups. The results demonstrated a significantly higher occurrence of severe and moderate bleeding in the placebo group compared to the SPGB group. At 30 and 60 minutes after the SPGB, there was a significant difference in bleeding as evaluated by the Boezaart scale between the 2 groups. This suggests that the SPGB had a beneficial effect in reducing bleeding during these time intervals. The lower incidence of bleeding in the SPGB group indicates that the specific intervention employed in the study may have contributed to better bleeding control. However, it is important to note that at the 90-minute mark, no significant difference in bleeding was observed between the SPGB and control groups based on the Boezaart scale. This finding suggests that the effect of the SPGB on bleeding might have diminished or reached a plateau beyond the 90-minute timeframe. Other factors, such as the natural course of surgical recovery and the body's hemostatic mechanisms, might have influenced bleeding outcomes during this later stage.
Furthermore, our study revealed a significant difference in the total bleeding amount between the intervention and control groups, with lower total bleeding observed in the SPGB group compared to the placebo group. This finding suggests that the intervention, in this case, SPGB, had a notable impact on reducing overall bleeding during the surgical procedure. By targeting the sphenopalatine ganglion, the SPGB technique may have contributed to improved hemostasis and reduced blood loss. The significant difference in total bleeding amount further supports the notion that the SPGB intervention played a role in minimizing bleeding, potentially through its effects on local blood vessels and coagulation mechanisms. By reducing blood loss, the SPGB technique may have numerous benefits, including facilitating a clearer surgical field, minimizing the need for transfusions, and improving overall patient outcomes. Consistent with our results, Sari and Uysal demonstrated that SPGB led to a significant reduction in bleeding and edema following septorhinoplasty (14). Ekici and Alagoz assess the impact of bilateral endoscopic SPGB on postoperative pain management in patients who have undergone septoplasty. The findings revealed that the SPGB group exhibited significantly reduced need for analgesics and reported higher levels of satisfaction regarding their pain control at both the 24- and 168-hours following surgery, in comparison to the control group. Furthermore, there was a significant difference between the 2 groups in terms of the amount of blood lost during the surgery, and the SPGB group experienced a longer surgical duration compared to the control group (17).
The mechanisms underlying the reduction of congestion in the context of bupivacaine administration involve its interaction with prostaglandin receptors and sodium pump blockade. Bupivacaine is a local anesthetic medication commonly used to provide pain relief and reduce inflammation in various medical procedures (21). Inside the nose, there are numerous small blood vessels that can become engorged and swollen when the immune system is activated in response to harmful factors. This increased blood flow leads to nasal congestion and difficulty in breathing through the nose. Additionally, the activation of the immune system triggers the production of excess mucus by the mucous glands inside the nose, further exacerbating the congestion (22, 23). Bupivacaine exerts its pharmacological effects by binding to prostaglandin receptors, specifically the prostaglandin receptor subunit EP1 (PGE2/EP1). The EP1 subunit is involved in various physiological processes, including the constriction of bronchioles and blood vessels. By binding to and inhibiting this receptor subunit, bupivacaine induces vasoconstriction, narrowing the blood vessels and reducing blood flow to the area. This decreased blood flow contributes to the reduction of swelling and congestion in the nasal passages (22, 24, 25). The binding of bupivacaine to prostaglandin receptors is also associated with its additional analgesic effects. By inhibiting the EP1 subunit, bupivacaine can alleviate pain, reduce inflammation, and potentially lower fever. These effects are attributed to the modulation of prostaglandin-mediated signaling pathways involved in pain perception, inflammation, and vasodilation (26, 27).
It is important to consider the limitations of this study. The sample size may have been limited, which could have affected the statistical power and generalizability of the results. The availability of eligible individuals for rhinoplasty procedures was limited, which resulted in a prolonged duration of sampling. A larger sample size might have provided more robust findings and better elucidated the potential differences between the groups. However, our research had notable strengths, including the comprehensive investigation of diverse variables associated with pain scores, bleeding, satisfaction levels, and clinical factors.
5.1. Conclusions
In conclusion, our study demonstrated significant reductions in intraoperative bleeding and pain in the SPGB group compared to the placebo group. Additionally, there was a significant difference in the average amount of fentanyl received between the SPGB and placebo groups, with the SPGB group requiring lower amounts of fentanyl. This finding suggests a potentially reduced need for opioids in intraoperative pain management and highlights the benefits of SPGB in reducing bleeding and minimizing opioid consumption. It also suggests that SPGB can improve pain control while mitigating the risks and side effects associated with opioids, making it an optimal choice for anesthesiologists aiming to reduce anesthesia drug consumption.
Overall, our findings support the effectiveness of combining the specific medication with SPGB to enhance surgical outcomes when the block is necessary. However, further research with larger sample sizes and comprehensive assessments of analgesic requirements is warranted to strengthen these findings and provide a more comprehensive understanding of the impact of SPGB on bleeding and pain in rhinoplasty and septoplasty surgeries.