1. Background
A C-shaped hollow tube resembling the cartilage tube, known as the trachea, is a vital component of the respiratory system (1). When circumferential defects exceed 50 mm, tracheal support is required for reconstructing the underlying structure, adhering to the anatomical and physiological characteristics of the body (2). Tracheal reconstruction or transplantation becomes necessary following prolonged intubation or in cases of autoimmune diseases leading to tracheal stenosis, tracheomalacia, and bronchomalacia. Methods for tracheal reconstruction encompass tracheal allografts, tracheal autografts, and tracheal prostheses.
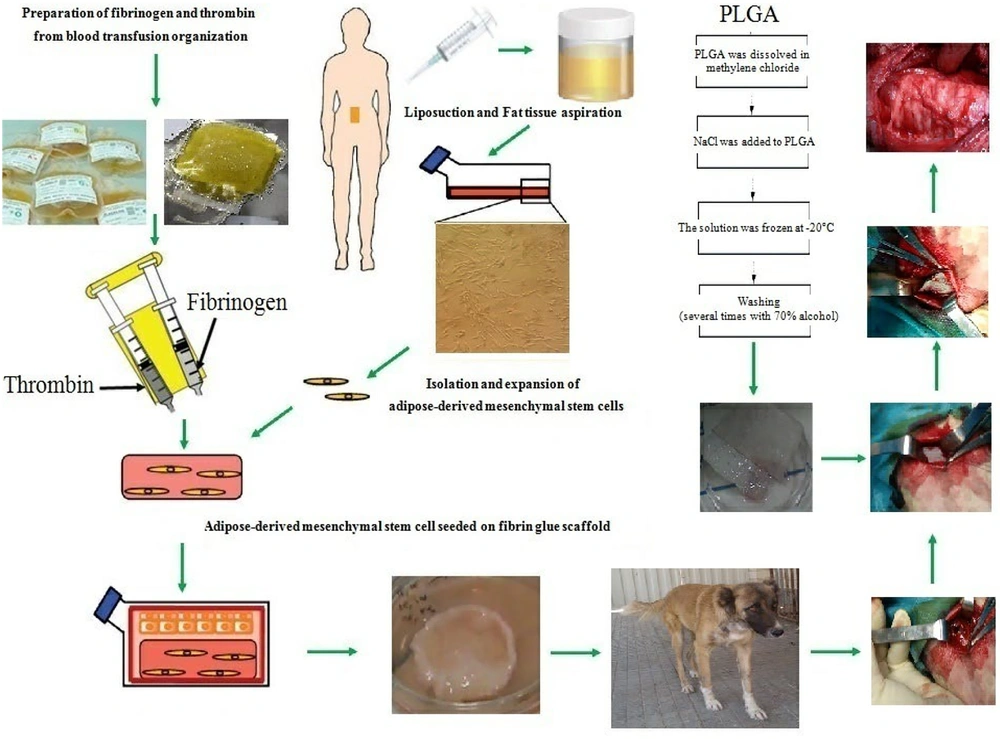
In this study, human adipose stem cells (HASCs) sourced from bone marrow and adipose tissue, along with biomaterials comprising synthetic and natural polymers, were utilized in tissue-engineered trachea construction (3). Human adipose tissue-derived stem cells (HADSCs) are undifferentiated cells with the capability to replenish cell populations and differentiate into various specialized cell types of their tissue origin.
Currently, it is well-established that these cells can undergo extensive differentiation into mesoderm-derived cell types when induced in vitro. While initially identified predominantly in bone marrow, ADSCs have since been found in other adult tissues such as the liver, umbilical cord blood, placenta, and dental pulp. Adipose tissue-derived stem cells can be cultured over extended periods without compromising their differentiation potential (4-10). Despite their robustness and ability to survive freezing temperatures, they exhibit diminished viability, proliferative capacity, and differentiation potential. Nonetheless, the most notable feature of ADSCs is their immunomodulatory capability, allowing transplantation with or without human leukocyte antigen (HLA) matching between donor and host. They express the major surface antigen, MHC-I (11, 12). In vivo experiments have confirmed the validity of the in vitro findings. Several studies have utilized xenografts of ADSCs and ADSCs isolated from humans due to the ease of tissue access and availability of lipoaspirate. Additionally, mice, rats, rabbits, and dogs have been utilized as recipients in various studies (13).
When developing three-dimensional biomaterials with high biocompatibility, the selection of a suitable scaffold for efficient tissue engineering of the trachea is crucial (14). Large-scale production of synthetic polymers is possible without the risk of viral infection. Fibrin plays a crucial role in wound healing, homeostasis, and cell migration due to its natural scaffold supporting cell migration. The addition of thrombin induces fibrin polymerization, making it suitable as an injectable scaffold. Fibrin has also been shown to stimulate the synthesis of elastin and collagen, induce remodeling and regenerative responses in many cell types, and increase the mechanical strength of scaffolds. The use of fibril scaffolds can promote collagen synthesis, organization, and fibril orientation in various cell types. Additionally, fibrin possesses several characteristics, such as cell adhesion, biocompatibility, and biodegradability, making it a viable option for stem cell isolation and delivery (15, 16).
Studies have revealed that F/I-NP scaffolds promote the chondrogenic differentiation and cartilage-specific gene expression of HADSCs (17). In vitro studies have demonstrated that HADSCs combined with avocado and unsaponified soybeans in fibrin glue/polylactic-glycolic acid (FG/PLGA) compositions enhance the expression of cartilage-specific genes in both tissues (18).
2. Objectives
In a canine model, we investigated the role of both biological and synthetic constructs (FG/PLGA) in tracheal regeneration.
3. Methods
For cell culture, DMEM was purchased from Sigma, while fetal bovine serum (FBS) and penicillin-streptomycin were obtained from Gibco.
3.1. Isolation and Expansion of Human Adipose-Derived Stem Cells
This study was conducted following ethical standards and received approval from the Ethics Committee of the Institutional Research Committee (Tehran University of Medical Sciences) (code: 56325, number: (IR.TUMS.IKHC.REC.1401.290). The research took place at a veterinary facility in Qom province.
Human liposuction aspirates were collected from three patients at the Department of General Surgery, Nekuei Hedayati Forqani Hospital, who underwent dermal lipectomy after providing informed consent. All patients completed the survey and signed a confirmation. Adipose tissue was briefly stored in phosphate-buffered saline (PBS) at 4°C upon transfer to the laboratory. Subsequently, the tissue was incubated overnight in this medium, washed multiple times with buffered saline, and then cut into small pieces. Following that, it was enzymatically digested using type I collagenase (0.15 mg/g adipose tissue) for 45 minutes at 37°C and centrifuged at 1400 rpm for 8 minutes. The resulting cell pellets in complete medium were transferred to cell culture flasks and incubated at 37°C with 5% CO2 and 95% humidity. The cell culture medium was changed every 3 days. After trypsinization, cells were transferred to passage 3 and used for differentiation (19).
3.2. Characterization of Human Adipose-Derived Stem Cells
After trypsinization, we examined the surface marker expression of CD44, CD90, CD105, and CD34 to determine the characteristics of HADSCs using flow cytometry.
3.3. Preparation of Human Fibrinogen and Thrombin
Fresh frozen plasma (FFP) and fibrinogen were prepared by the Qom Branch Transfusion Organization. Thrombin was separated from FFP by centrifugation at 2200 rpm. The method involved adding calcium gluconate at a ratio of FFP 3:5, followed by incubation at 37°C for 50 minutes. After centrifugation, the supernatant was removed. The combination of fibrinogen and thrombin may enable the differentiation of cells as scaffolds.
3.4. Preparation of PLGA Scaffold
We dissolved 5% PLGA particles in methylene chloride (CH2Cl2) and mixed with NaCl. Then, a glass mold (height 5 mm, length 15 mm, width 15 mm) was filled with the polymer solution/NaCl particles and frozen at -20°C for 2 days. The frozen polymer scaffolds were placed in distilled water for 48 hours, with the water being changed every 2 hours to remove particles. Finally, the samples were sterilized using UV. The membranes were then incubated in DMEM medium for 2 hours, and cultured human adipose tissue stem cells (2 × 10) from the second passage (P3) were trypsinized and seeded onto the scaffolds.
3.5. Chondrogenic Differentiation
After three passages, HADSCs were suspended in fibrinogen at a cell density of 15 × 106 cells/mL. The mixture of cells and fibrinogen was then added to thrombin in chondrogenic medium containing DMEM-high glucose supplemented with 50 mg/mL bovine serum albumin, 5 μg/mL ascorbic acid-2-phosphate, 1% insulin Transferrin-selenium, 1 nM dexamethasone, 5 μg/mL linoleic acid, 1% penicillin-streptomycin, and 10 ng/mL transforming growth factor-β3, and incubated for 2 weeks at 37°C, 5% CO2, and 99% humidity
3.6. Implantation of DHASCs/FG/PLGA Into Tracheal Defect
Two days before the surgery, the canines (6 animals with a mean age of 12 months) consumed 20 mg/kg prednisolone every day by injection in accordance with the ethical standards of the institutional and/or national research committee. At this stage of the study, after fasting for 14 hours, the animals were anesthetized with ketamine 6 mg/kg and xylazine 2 mg/kg, the skin was disinfected, and the neck area was incised, followed by an incision through the three cartilaginous rings of the trachea with dimensions 30 × 10 mm, 10 × 10 mm, and 10 × 10 mm were removed, and implanted with DHASCs/FG/PLGA (group 1), FG/PLGA (group 2) and PLGA/SNS (group 3), respectively and then their accretion were performed in 6-0 polypropylene sutures. Randomly, each 6 dogs was allocated to 3 groups (2 in each group) because animals as samples were in the same situation, and it was not necessary.
After the operation, 500 mg amikacin and prednisolone injections were continued for two months once a day, and the daily use of prednisolone was minimized by a gradual reduction of the dosage (from 20 mg/kg to 5 mg/kg). Prednisolone was used for its potent anti-inflammatory and immunosuppressive activities.
After 2 months, all 6 animals were anesthetized, and the samples from the repaired sites, as well as the natural tracheal cartilage tissues, were harvested. Following the overdose, they consumed 30 mg/kg of ketamine and Xylazine 10 mg/15 kg (0.38%) of both.
3.7. In Vivo and In Vitro Real-time-Polymerase Chain Reaction for Evaluating the Expression of Chondrogenic Genes
After 2 weeks of chondrogenic differentiation and 2 months after surgery (biopsy), samples containing: In vitro groups: Chondrogenic DHASCs/FG and HASCs/FG and in vivo groups: DHASCs/FG/PLGA (group 1), FG/PLGA (group 2) and PLGA/SNS (group 3) were washed with PBS and RNA isolation was performed separately for both groups. Both groups were then digested in liquid nitrogen (Table 1).
| Primer | Sequences (5' → 3') |
|---|---|
| Collagen type 1 | |
| Forward | TTGTACAGACATGACAAGAGGC |
| Reverse | CTCTACCTGGGTACTACCCA |
| Aggrecan | |
| Forward | CAGAGTGAAATCCACCAAGT |
| Reverse | TGTCCGTGGACAAACAGGTA |
| Sox9 | |
| Forward | TACGACTACACGCACCACCA |
| Reverse | TTAGGATCATCTGCGCCATC |
| Collagen 2 | |
| Forward | ACACAGCGCCTTGAGAAGAG |
| Reverse | TTCTACGGTCTCCCCAGA GA |
3.8. Glycosaminoglycan Assay
Glycosaminoglycan (GAG) production was assessed using a dimethyl methylene blue (DMMB) assay as previously described. Repaired tracheal cartilage samples were digested with 100 μL of 50 μg/mL proteinase K in 0.1 M K2HPO4 (pH 8.0) overnight at 56°C. After inactivating the enzyme at 90°C for 10 minutes, the samples were centrifuged at 14000 g for 10 minutes, and each supernatant was collected for GAG and DNA quantification.
The samples were then incubated at room temperature in 0.04 M glycine/NaCl (pH 3) with 16 mg/mL of DMMB, and the absorbance was read at 500 nm using the ELx800 plate reader (Biokit, ELx800 Reader, Spain). The amount of GAGs was determined relative to known concentrations of chondroitin sulfate (Sigma-Aldrich) and normalized to the total DNA content determined below. Additionally, 0.2 μg/mL Hoechst 33258 was added to the samples for 1 min at room temperature, fluorescence was measured (excitation 340 - 370 nm, emission 440 - 460 nm), and the DNA concentration was compared to that of each sample. The standard curve utilized salmon sperm DNA.
3.9. In Vitro and In Vivo Histological Examination
For in vitro evaluation, samples were harvested after 14 days, and for in vivo examination, biopsy specimens from the tracheal defect site were obtained after 8 weeks and transferred to the laboratory. They were washed with PBS, fixed in 10% formalin for 24 h, dehydrated in various concentrations of ethanol, and embedded in paraffin. Subsequently, 5 μm sections were prepared and stained with hematoxylin and eosin (H&E) for histological examination.
3.10. Immunohistochemistry
Eight weeks after implantation, immunohistochemistry was performed on the samples. Samples were washed three times with 0.1 M PBS and fixed in 10% formalin overnight. After fixation, the samples were embedded in paraffin and sectioned with a microtome into 5-μm-thick sections. After the antigen retrieval, the non-specific antibody binding site was blocked with a blocking buffer. The phenotypic expression of these sections was examined through the implementation of immunocytochemical staining accompanied by antibodies against human antigens type II collagen (ab3092; Abcam). Briefly, fixed sections were washed three times with cold PBS. After washing with PBS, the primary antibody described above was added, and the slides were left overnight at room temperature. The next day, the primary antibody was removed by washing three times with PBS, a secondary antibody conjugated to horseradish peroxidase (anti-mouse IgG secondary antibody (ab2891; Abcam)) was added, and the slides were stored overnight at room temperature. It was then developed using DAB (3, 3'-diaminobenzidine) substrate kit (ab94656; Abcam). Human articular cartilage was readied using the same method as positive controls of collagen type II.
3.11. Statistical Analysis
In vitro, real-time polymerase chain reaction (PCR) analysis confirmed significant differences in the expression levels of four genes in the two groups by t-test, P ≤ 0.05. Meanwhile, the comparison of gene expression between different groups in vivo was performed by two-way analysis of variance (ANOVA) and post hoc least significant difference (LSD) test. Additionally, the GAG assay was analyzed using Duncan's multirange test. Real-time PCR was repeated four times for each sample. Data were analyzed using SPSS software (version 17), and data were measured with P-value < 0.05. All data were expressed as mean ± standard error.
4. Results
4.1. Proliferation of Adipose-Derived Stem Cells
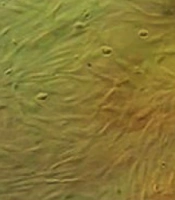
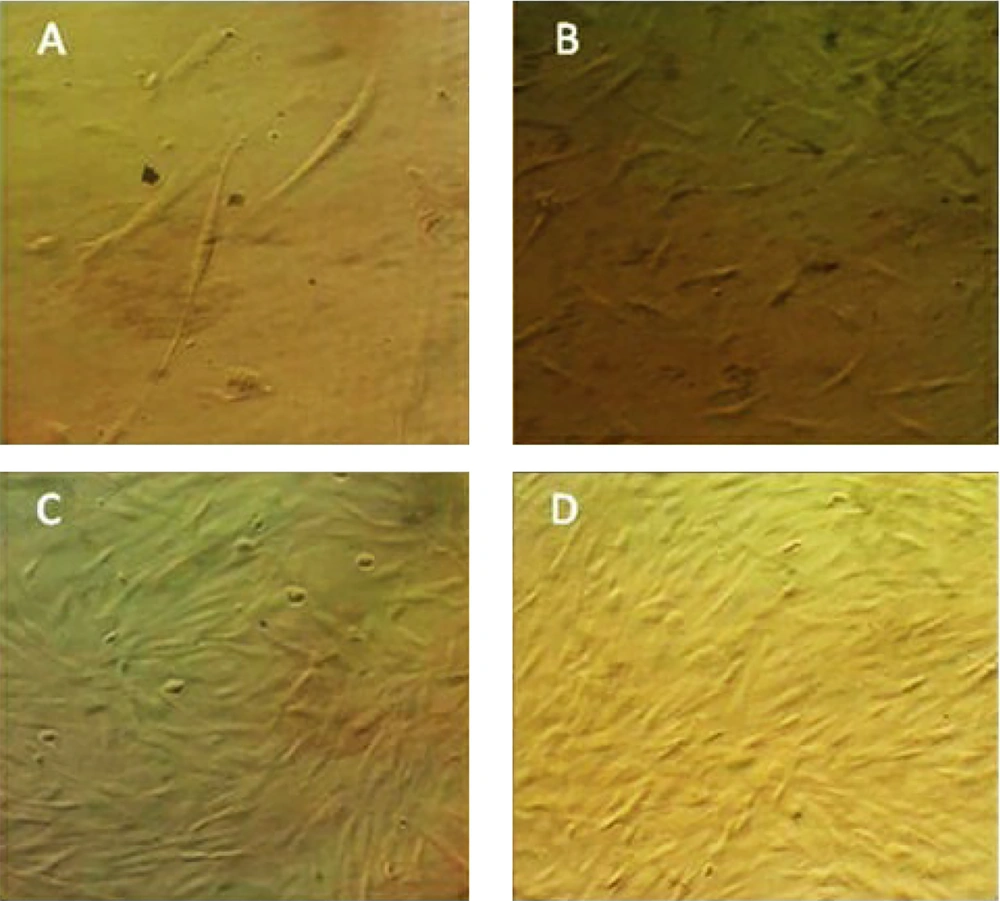
In the current study, human adipose-derived stem cells exhibited a fibroblast-like appearance when observed under a phase-contrast microscope. The cells displayed homogeneous and uniform characteristics, with proliferation observed in passage 3 (Figure 1).
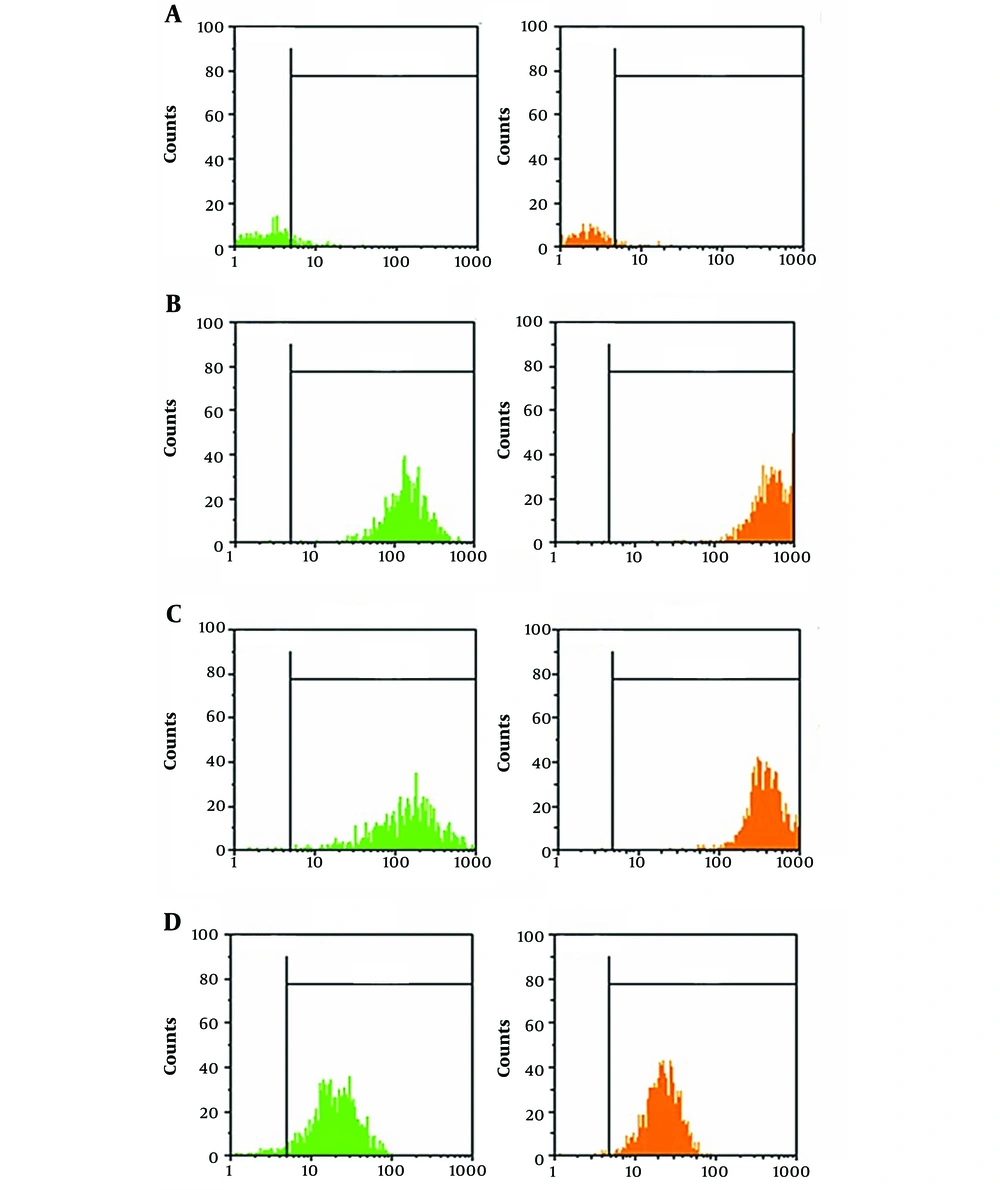
4.2. Flow Cytometry Evaluation
To characterize the adipose-derived stem cells, various markers associated with HADSCs were considered, including CD34, CD44, CD90, and CD105 (Figure 2).
4.3. Evaluation of the Chondrogenic Genes Expression In Vitro and In Vivo by Real-time PCR
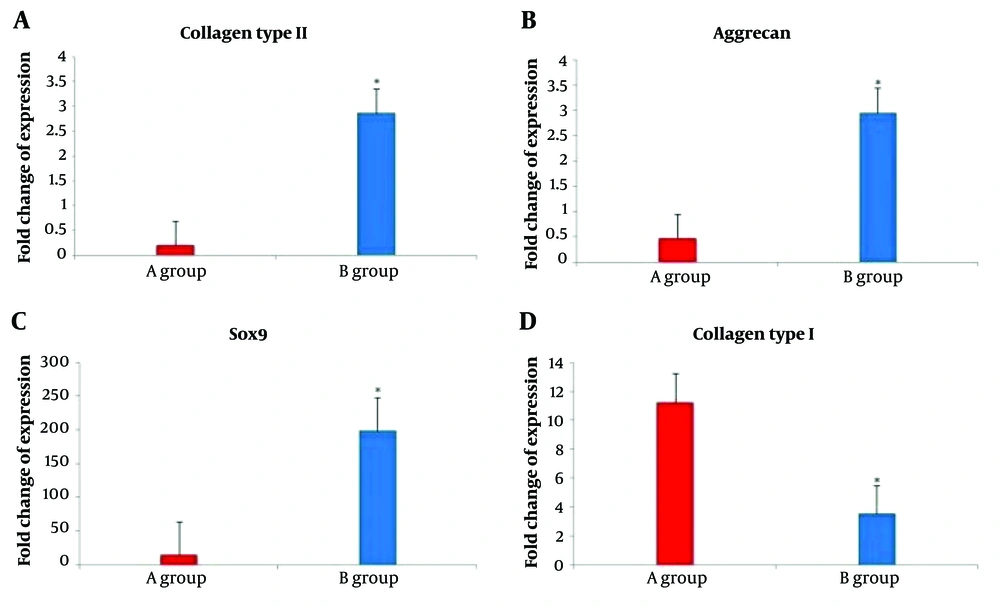
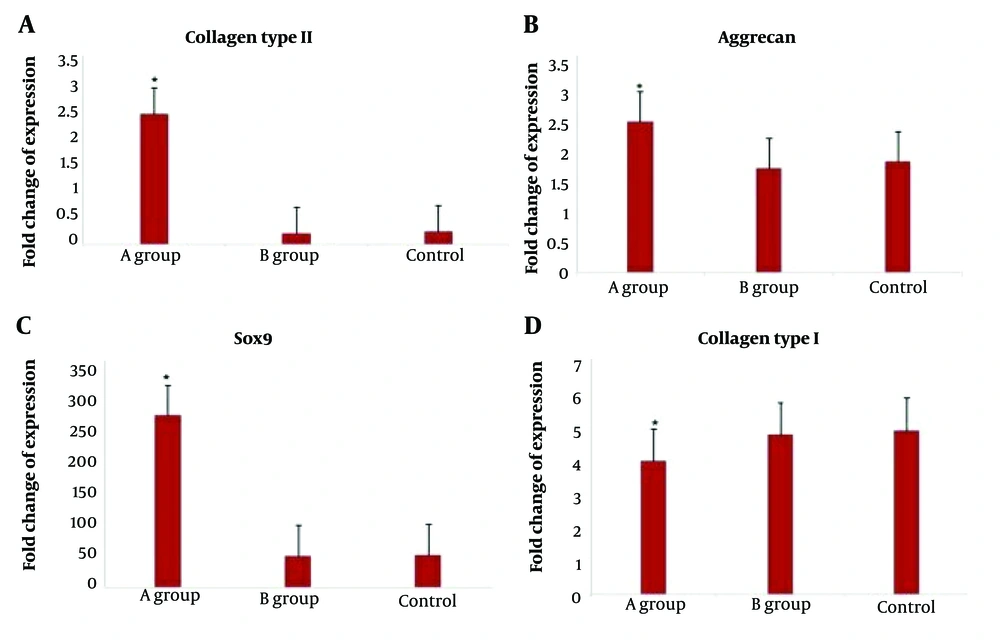
Expression of collagen type I and II, sox 9, and aggrecan was demonstrated in the in vitro groups (Figure 3) and after 8 weeks in the implantation groups (in vivo), as confirmed by real-time PCR results (Figure 4).
Real-time polymerase chain reaction (PCR) results demonstrating the expression of chondrocyte-specific markers by human adipose-derived stem cells after induction with chondrogenic medium in vitro. A, type II collagen; B, aggrecan; C, Sox9; and D, type I collagen. Two groups contained chondrogenic human adipose stem cells/fibrin glue (HASCs/FG) (A); differentiated HASCs (DHASCs)/FG (B).
Real-time polymerase chain reaction (PCR) results demonstrating the expression of chondrocyte-specific markers by human adipose-derived stem cells after induction with chondrogenic medium in vivo. A, type II collagen; B, aggrecan; C, Sox9; and D, type I collagen. Two groups contained polylactic-glycolic acid/sterile normal saline (PLGA/SNS) (control group A), fibrin glue (FG)/PLGA (group B), and differentiated human adipose stem cells (DHASCs)/FG/PLGA (group control).
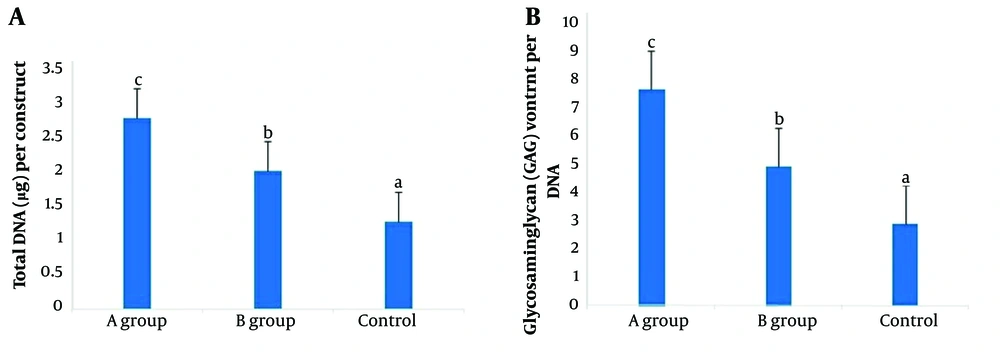
4.4. GAG Quantification
Glycosaminoglycans are major components of the extracellular matrix and play crucial roles in various tissues and organs. The GAG density data revealed a significant difference between the DHASCs/FG/PLGA group and the other groups (Figure 5).
4.5. Evaluation of the Histological Characteristic In Vitro and In Vivo
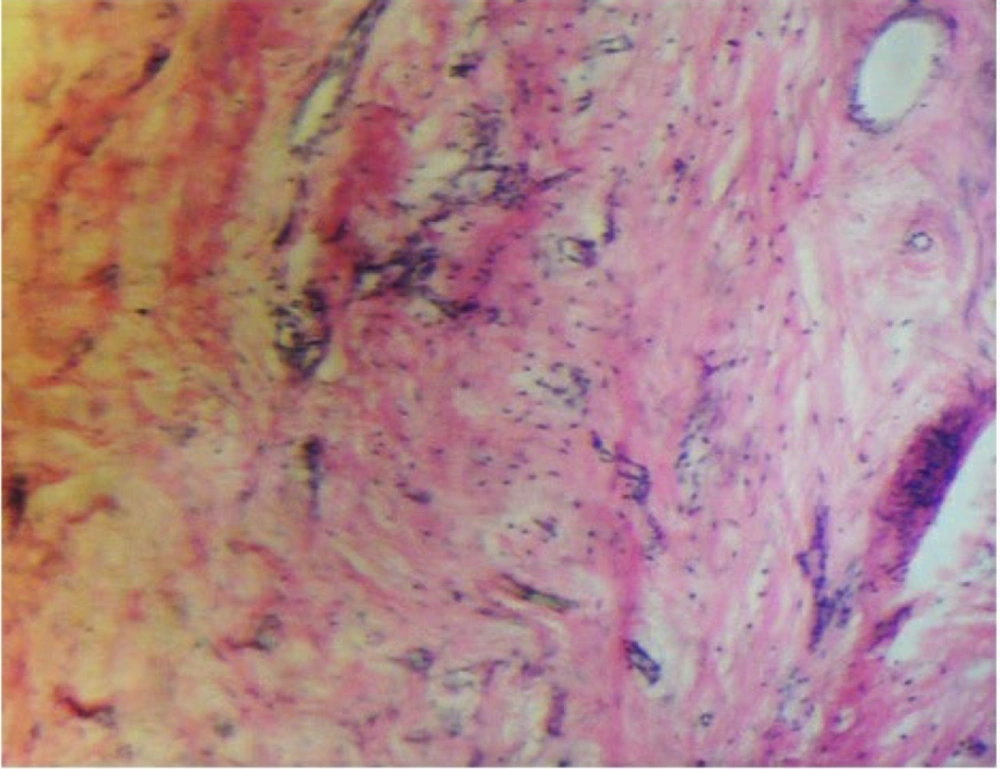
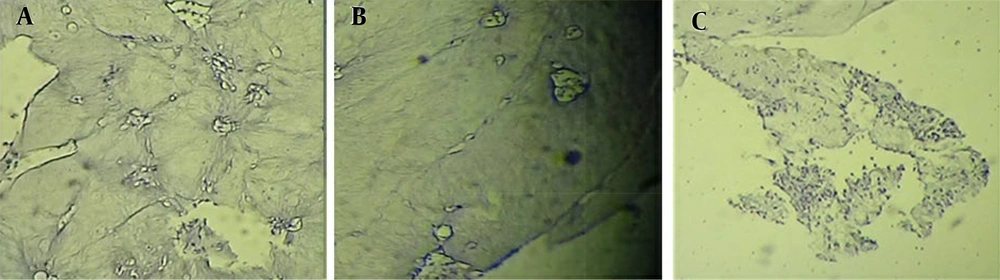
To confirm the presence of chondrocytes, samples were examined using hematoxylin/eosin staining after 2 weeks in the in vitro groups (Figure 6) and after 8 weeks in the transplanted groups (Figure 7). The presence of chondrocytes within lacunae indicated cartilage tissue formation in the injured area of the trachea, particularly in the DHASCs/FG group (in vitro) and DHASCs/FG/PLGA group (in vivo). However, FG/PLGA and PLGA/SNS groups within the defect area did not result in cartilage formation. An overview of the research is provided in Figure 8.
Hematoxylin and eosin staining demonstrated cartilage tissue in the defect of tracheal rings in A, differentiated human adipose stem cells (DHASCs)/fibrin glue (FG)/polylactic-glycolic acid (PLGA) groups; while in the B, FG/PLGA groups; and C, PLGA/sterile normal saline (SNS) groups (control); cartilage is not formed (in vivo).
4.6. Immunohistochemistry
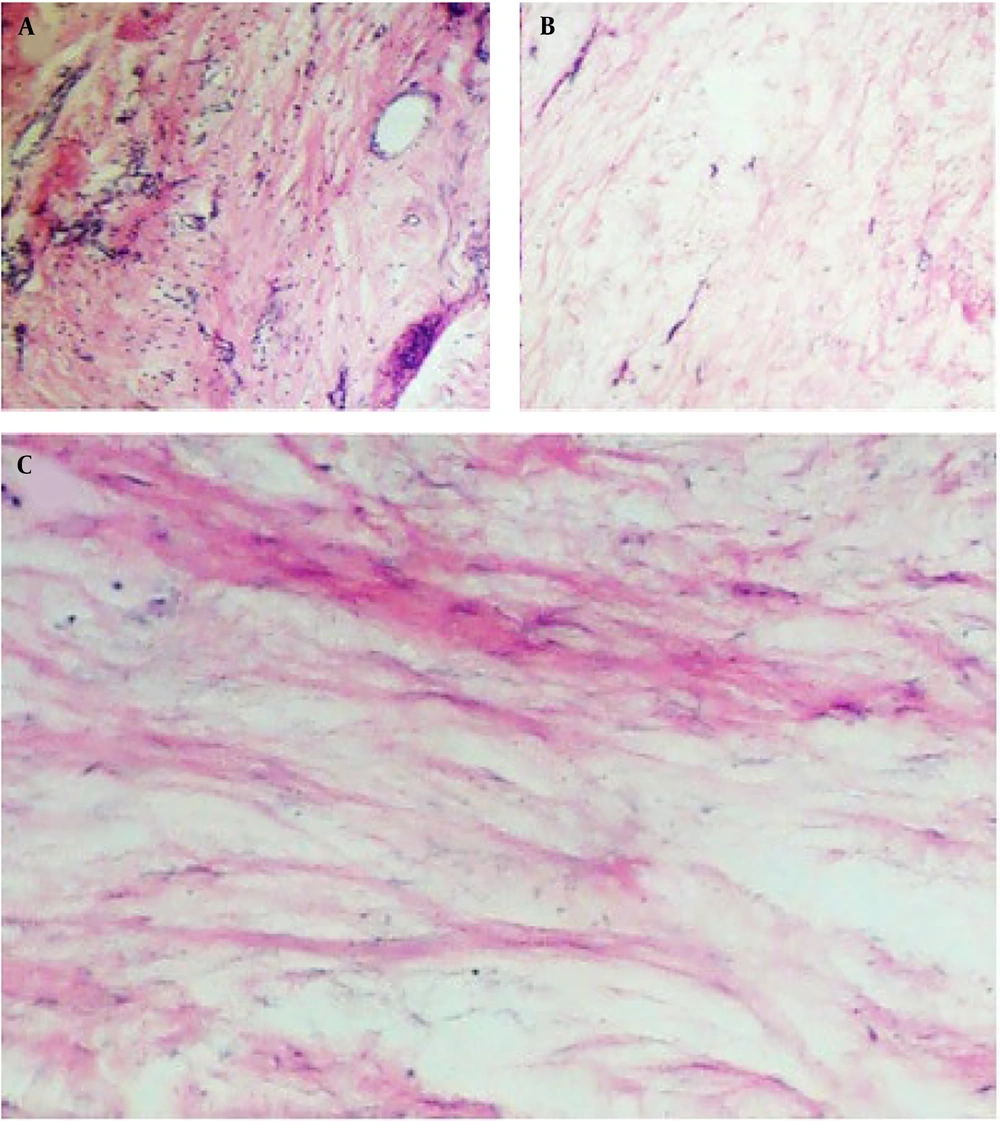
Immunohistochemical analysis demonstrated significant deposition and accumulation of extracellular matrix components in the intervertebral disc, particularly collagen type II, the major collagen of articular cartilage. Collagen type II was evenly distributed in group 1 (Figure 9), whereas in other groups (e.g., human articular cartilage as a control), the distribution of collagen type II was less uniform.
Immunohistochemical analysis showed that type II collagen was uniformly distributed in the differentiated human adipose stem cells (DHASCs)/fibrin glue (FG)/polylactic-glycolic acid (PLGA) groups, but in the other groups, type II collagen distribution was less homogenous. A, DHASCs/FG/PLGA groups; B, FG/PLGA groups; and C, PLGA/sterile normal saline (SNS) (control groups).
5. Discussion
The results of our study demonstrate that xenogeneic HASCs transplantation holds promise for improving tracheal defects. In vitro studies using real-time PCR and histological tests revealed the expression of chondrogenic markers such as collagen II, collagen I, sox9, aggrecan, and the presence of chondrocytes. Similarly, in vivo studies conducted after 8 weeks showed chondrogenic gene expression and the formation of cartilage in the tracheal defect area. The tracheal repair was achieved by encapsulating differentiated chondrocytes from HASCs in FG/PLGA, whereas the use of PLGA scaffold alone failed to address the defect area. Tracheal stenosis and congenital tracheal diseases continue to present challenges in tracheal reconstruction across various tracheal-related conditions (20).
The immunopurified nature of HASCs, characterized by low levels of cell surface class I and the absence of costimulatory molecules such as CD40, CD40L, CD80, and CD86, inhibits recognition by HLA class II and T lymphocytes. This characteristic enables these cells to evade immune system detection (21-23). Our findings indicate that human adult stem cells possess low immunogenicity and can suppress the function of mature T cells. Despite upregulating MHC class I and II molecules in the presence of interferon-γ (INF-γ), they do not elicit immunological responses. However, under inflammatory conditions, MSCs have been shown to upregulate the costimulatory molecule CD40 (24, 25).
While autologous cells offer the advantage of avoiding rejection and reducing infection risks, their use is also associated with drawbacks such as delays between cell collection and transplantation, leading to patient harm, as well as logistical challenges in transporting cells to the laboratory for quality control, donor cell isolation, and expansion. Tolerogenic allogeneic or xenogeneic adult stem cells are preferred alternatives as they obviate the need for cloning frozen donor cells and provide a potentially unlimited therapeutic supply (25-30).
Xenotransplantation, which involves using animals as a source of organs and cells, offers several advantages, such as the potential for unlimited generation of organs, tissues, and cells. This can facilitate transplantation procedures in individuals with limited options and increase the availability of organs (31). The first study on intracerebral transplantation of human adult stem cells was conducted in 2002, demonstrating improved neurological function in a rat cerebral ischemia model (32). Subsequent research showed that both undifferentiated and osteogenic-differentiated adult stem cells (ADSCs and BMSCs) could survive for extended periods when injected into immunocompetent mice, with undifferentiated cells proving to be particularly suitable for xenotransplantation. Additionally, insulin-producing cells differentiated from human adult stem cells were found to survive long-term when transplanted into streptozotocin-induced diabetic mice (33, 34).
Xenotransplantation is a medically regulated procedure that requires further research to mitigate risks and enhance efficacy. Studies have explored composite scaffolds, such as bone matrix gelatin and fibrin glue, which have shown promise in preserving cellular morphology, promoting proliferation and attachment, and facilitating extracellular matrix synthesis (35). Recent research has also identified optimal concentrations of fibrin and thrombin for cartilage regeneration, with high fibrin concentrations resembling native chondrocyte morphology and leading to the highest levels of cartilage formation at 4 weeks post-implantation (36). Continued investigation in this field is crucial for refining xenotransplantation techniques and improving patient outcomes. In an animal model, the functional regeneration of tracheal epithelial tissue was observed through the use of insulin-like growth factor-1 (IGF-1) and basic fibroblast growth factor (bFGF), hepatocyte growth factor (HGF), and epidermal growth factor (EGF) in a collagen sponge scaffold (37). In another laboratory study, a laser method was employed to decellularize the natural matrix tissue of the trachea. The results demonstrated that the level of cell adhesion was maintained with complete biocompatibility, suggesting it is a promising foundation for tracheal repair research (38). This study showed that differentiated chondrocytes from HASCs encapsulated in fibrin glue could serve as suitable candidates for repairing tracheal cartilage defects in vivo. However, further research involving a larger number of animals is necessary before fibrin glue/differentiated human adult stem cells can be clinically applied for cartilage engineering.
5.1. Conclusions
In conclusion, the findings suggest that heterologous factors can be considered a novel technique for reconstructing and repairing damaged areas. These factors have the potential for commercial use as active ingredients in pharmaceuticals.