1. Background
Temporal lobe epilepsy (TLE) is the most common type of focal epilepsy, characterized by frequent resistance to antiepileptic drugs (AED). Its significantly more frequent cause is hippocampal sclerosis, and it is tightly associated with cognitive difficulties (1). This is further amplified by the critical role the temporal lobe (LT) plays in cognitive function, making it the brain’s most epileptogenic region (2). Consequently, multiple neuropsychological deficits are often present in TLE patients, even at diagnosis, significantly impacting their daily lives and overall quality of life (3).
Neuropsychological studies have identified several cognitive dysfunctions associated with TLE (4). Firstly, memory both short- and long-term memory can be affected; specifically, working memory dysfunction impairs visuospatial and verbal abilities (5). Additionally, TLE can significantly disrupt long-term memory, leading to substantial loss of autobiographical memories (6). Secondly, executive functions can be affected; although some studies have shown deficits in patients with temporal lobe epilepsy (PWTLE) (4), others report conflicting results (7). Further research is needed to clarify this relationship. Furthermore, language difficulties are diverse, including difficulties accessing the lexicon, leading to word-finding difficulties and semantic paraphasia. Additionally, slower response times in naming tasks with picture presentation or verbal fluency tests (8).
Presently, several studies point to the multifactorial nature of cognitive impairment in epilepsy, highlighting the influence of the underlying cause (4). One study by Vaccaro et al. investigated independent associations with cognitive performance in PWTLE and showed that years of education and male gender were positive predictors. Nevertheless, left laterality of TLE, longer duration of epilepsy, and polytherapy (multiple AEDs) were negatively associated with cognition (9). However, other studies report different findings. There were identified negative associations between cognitive performance in PWTLE and several factors, including early age of onset, higher seizure frequency, a greater number of AEDs, longer duration of illness, and the presence of generalized seizures (10, 11).
In addition, PWTLE frequently co-occurs with psychiatric comorbidities, such as anxiety and depression. Anxiety disorders affect 20% to 30% of PWTLE; however, depression rates range from 30 - 35% in general studies and can reach 50% in specialized epilepsy centers (9, 12). These conditions can negatively impact cognitive performance in PWTLE (13, 14). Low self-esteem is also reported in focal epilepsy patients and appears to influence cognition, although further research is needed to clarify this relationship (15).
However, studies investigating factors influencing cognitive performance in PWTLE present inconsistent findings. Several factors contribute to this difficulty: Methodological issues within studies, the inherent variability in individual cognitive abilities within specific cultures, and the diverse types of epilepsy with their unique pathophysiological processes (16).
The current study delves into the complex interplay of clinical, demographic, psychological, and social factors influencing cognitive abilities in low-income PWTLE residing in a predominantly rural region. This understudied population could, firstly, advance our understanding by examining the relative contributions of various factors to cognitive performance, and we can refine and expand our knowledge in this area. Secondly, developing targeted interventions by identifying specific factors impacting cognition paves the way for designing and implementing personalized support interventions to optimize cognitive function in PWTLE. Furthermore, this study can serve as a springboard for exploring other impactful factors and interventions to ultimately improve the overall well-being of PWTLE.
2. Methods
2.1. Study Design and Participants
This case-control analytical study was conducted between July 2021 and August 2022 at “the Day Clinic”, a public health care clinic in the city of Agadir, Morocco, offering neurological consultations to residents of Souss Massa Region, Morocco. This study evaluated a group of PWTLE (n = 40) and a control group (n = 92).
The participants, identified as patients diagnosed with TLE through established clinical protocols, were recruited consecutively. Diagnoses were grounded in seizure semiology and the presence of interictal epileptiform discharges (IEDs) in the electroencephalogram (EEG) indicative of TLE.
An extensive anamnesis was performed with each patient. Initial data collection obtained demographic characteristics, such as age, gender, educational level, occupation, income, marital status, and residential area. Subsequently, clinical details encompassing medical, surgical, and familial history, type of epilepsy, epilepsy laterality, age at epilepsy onset, epilepsy duration, and seizure frequency were documented. Furthermore, in PWTLE, we studied treatment and lifestyle characteristics, including dietary habits, sleep patterns, physical activity, social support, and memory-related complaints.
The inclusion criteria for all participants comprised: (a) low-income households (monthly household income below Morocco's ((guaranteed inter-professional minimum wage)): i.e., monthly income less than 277 US dollars); (b) age of 18 years or above; and (c) agreement to participate in the study.
The exclusion criteria were: (a) participants with concurrent severe neurological and/or psychiatric disorders; (b) illiterate individuals; (c) non-Arabic speakers; (d) those experiencing an epileptic seizure within 24 hours preceding the neurocognitive assessment; (e) previous brain surgery recipients; and (f) rejection to participate.
We selected a control group (n = 92) from the same sociocultural background as the PWTLE (families and companions). The PWTLE and the control group were matched for gender, age, level of education, and residential area (Table 1).
| Sociodemographic Characteristics of Participants | PWTLE b (n = 40) | Control Group b (n = 92) | t/χ2 | P c |
|---|---|---|---|---|
| Gender (men) | 23 (57.5) | 47 (51.1) | 0.46 | 0.497 |
| Age | 33.35 ± 14.27 | 35.37 ± 14.61 | 0.735 | 0.464 |
| Educational level (high school and university) | 21 (52.5) | 53 (57.6) | 0.295 | 0.587 |
| Residential area (rural) | 16 (40) | 35 (38) | 0.045 | 0.839 |
| Marital status (single) | 30 (75) | |||
| Occupation (employed) | 10 (25) |
Abbreviations: PWTLE, patients with temporal lobe epilepsy; SD, standard deviation.
a Values are expressed as No. (%) or mean ± SD.
b P indicates the P-value for comparing different categories. The P-value was calculated using the Student test (t) or Pearson chi-square test (χ2), as appropriate.
c Statistically significant at P < 0.05.
2.2. Data Collection
2.2.1. Evaluation of Cognitive Functions
Cognitive functioning was assessed by employing the Montreal cognitive assessment (MoCA) -Arabic version (17). The MoCA has demonstrated an increased pathological finding in TLE patients (18). This instrument covers multiple domains: Attention, concentration, executive functions, episodic memory, language, visual constructive praxis, abstraction, calculation, and orientation. Its administration generally varies from 15 to 20 minutes. A MoCA score ≥ 26 indicated normal cognitive function.
2.2.2. Evaluation of Anxiety and Depression
The Hospital Anxiety and Depression Scale (HADS) - Arabic version - (19) was deployed to assess anxiety and depression levels. It allows the identification of these disorders, excluding somatic symptoms, which could prevent an accurate assessment. Renowned for its efficacy and brevity, the HADS can be administered in 2 to 6 minutes and has proven validity in epileptic patients.
2.2.3. Evaluation of Self-esteem
The Rosenberg Self-Esteem Scale (RSES) - Arabic version - (20) was employed to assess participants' self-esteem. The overall score ranges between 10 and 40, with higher scores suggesting higher self-esteem.
2.2.4. Statistical Analysis
To ensure that the two groups (PWTLE and control) were matched for age, gender, education level, and residential area, we compared them using the Student test or Pearson chi-square test, as appropriate. Statistical analyses were performed for all variables. Furthermore, the descriptive analysis calculated frequencies and proportions for categorical variables. Additionally, means with standard deviation (SD) or median with interquartile range (IQR) were calculated for continuous variables.
The Kolmogorov-Smirnov test was used to assess the normality of the data. The comparison of cognitive function between two groups (PWTLE and control) was analyzed using the Student test. Spearman's correlation test was used to examine factors correlated with cognitive function. The factors affecting cognitive function were assessed by univariate logistic regression analyses. The variables showing statistical significance at a level of (P = 0.1) were subjected to multivariate logistic regression analyses. Data analysis was performed using SPSS 25.0 for Windows. A significance level of P < 0.05 was considered.
2.2.5. Ethical Considerations
It was affirmed that this report aligns with the ethical publication guidelines endorsed by the journal. The research protocol received approval from the Ethics Committee for Biomedical Research of Rabat (CER) at the Faculty of Medicine and Pharmacy, Mohammed V University in Rabat, Morocco (reference CERB 84-21). All procedures involving human participants adhered to ethical standards established by institutional and national research committees and the 1964 Helsinki Declaration and its subsequent amendments. Informed consent letters were sent to the participants, ensuring confidentiality and anonymity.
3. Results
3.1. Sociodemographic and Clinical Characteristics of Participants
A total of (n = 40) PWTLE and (n = 92) controls were participated in this study. The average age of the PWTLE and control group was 33.35 (14.27) and 35.37 (14.61) years, respectively. Additionally, 57.5% and 51.1% of PWTLE and control groups were male, respectively. Moreover, 40% and 35% of PWTLE and control groups came from rural backgrounds, respectively. The sociodemographic and clinical characteristics of the participants are summarized in Table 1 and Table 2.
| Clinical Characteristics of Epilepsy | Values a |
|---|---|
| Age of onset of epilepsy | 14.5 [7.25 - 19] |
| Duration of epilepsy | 13 [6 - 22.75] |
| Seizure-free (one year or more) | 12 (30) |
| Handedness | |
| Left | 12 (30) |
| Right | 15 (37.5) |
| Mixed | 10 (25) |
| Unknown | 2 (5) |
| Polytherapy | 16 (40) |
| MRI (abnormality) | 8 (20) |
| Consanguinity | 7 (17.5) |
| Family history of epilepsy | 9 (22.5) |
| History of febrile seizure | 10 (25) |
| Memory complaint | 27 (67.5) |
| Depressive symptoms | 9 (22.5) |
| Anxiety symptoms | 18 (45) |
| Low self-esteem | 29 (72.5) |
Abbreviations: IQR, interquartile range; MRI, magnetic resonance imaging.
a Values are expressed as median [IQR] or No. (%).
3.2. Cognitive Test Scores of PWTLE and Control Groups
The study revealed that the average MoCA score in the PWTLE and control groups was 18.88 ± 8.17 and 25.78 ± 3.42, respectively. Furthermore, PWTLE showed low performance in the average MoCA and in the seven subtests in comparison to the control group (P < 0.05) (Table 3).
| Variables | PWTLE a (n = 40) | Control a (n = 92) | t | P b |
|---|---|---|---|---|
| MoCA general score | 18.88 ± 8.17 | 25.78 ± 3.42 | 5.154 | 0.000 c |
| Visuospatial/executive score | 2.45 ± 2.06 | 3.85 ± 1.34 | 3.939 | 0.000 c |
| Naming score | 2.4 ± 0.93 | 2.96 ± 0.2 | 3.752 | 0.001 c |
| Attention score | 3.35 ± 2.12 | 4.46 ± 1.54 | 2.977 | 0.004 c |
| Language score | 1.65 ± 0.9 | 2.48 ± 0.54 | 5.443 | 0.000 c |
| Abstraction score | 1.4 ± 0.84 | 1.77 ± 0.44 | 2.637 | 0.011 d |
| Delayed recall score | 1.85 ± 2.22 | 3.78 ± 1.23 | 5.157 | 0.000 c |
| Orientation score | 5.23 ± 1.44 | 5.85 ± 0.44 | 2.679 | 0.000 c |
Abbreviations: PWTLE, patients with temporal lobe epilepsy; SD, standard deviation; MoCA, Montreal cognitive assessment.
a Values are expressed as mean ± SD.
b The P-value was calculated using the Student test (t).
c P < 0.01
d P < 0.05.
3.3. Variables Associated with the Cognitive Functions of PWTLE
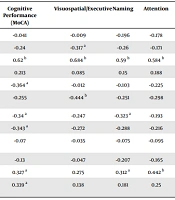
A strong correlation (r = 0.62, P < 0.01) was observed between higher MoCA scores and a higher level of education. In addition, a significant correlation was noticed between MoCA scores and several other factors, including seizure frequency, polytherapy, disease duration, and self-esteem scores (r = -0.364, P < 0.05), (r = -0.34, P < 0.05), (r = -0.343, P < 0.05), and (r = 0.327, P < 0.05), respectively. Moreover, these variables exhibited several statistically significant correlations of varying intensity, ranging from low to moderate, with multiple MoCA subtests, including naming, attention, language, abstraction, and delayed recall. Furthermore, multiple correlations were identified between the MoCA subtests and some variables studied, as indicated in Table 4.
| Variables | Cognitive Performance (MoCA) | Visuospatial/Executive | Naming | Attention | Language | Abstraction | Delayed Recall | Orientation |
|---|---|---|---|---|---|---|---|---|
| Gender | -0.041 | -0.009 | -0.196 | -0.178 | 0.043 | 0.175 | 0.097 | 0.179 |
| Age | -0.24 | -0.317 a | -0.26 | -0.171 | -0.398 a | -0.079 | -0.209 | -0.093 |
| Educational level | 0.62 b | 0.684 b | 0.59 b | 0.584 b | 0.61 b | 0.461 b | 0.471 b | 0.528 b |
| Age of onset | 0.213 | 0.085 | 0.15 | 0.188 | 0.18 | 0.145 | 0.261 | 0.079 |
| Seizure frequency | -0.364 a | -0.012 | -0.103 | -0.225 | -0.102 | -0.396 a | -0.404 b | -0.271 |
| Generalized seizure | -0.255 | -0.444 b | -0.251 | -0.298 | -0.235 | -0.008 | -0.144 | -0.19 |
| Polytherapy | -0.34 a | -0.247 | -0.323 a | -0.193 | -0.283 | -0.34 a | -0.377 a | -0.268 |
| Disease duration | -0.343 a | -0.272 | -0.288 | -0.216 | -0.335 a | -0.221 | -0.465 b | 0.017 |
| Depression (HADS-D) | -0.07 | -0.035 | -0.075 | -0.095 | -0.08 | -0.218 | 0.006 | -0.137 |
| Anxiety (HADS-A) | -0.13 | -0.047 | -0.207 | -0.165 | -0.087 | -0.087 | -0.045 | -0.069 |
| Self-esteem (RSES) | 0.327 a | 0.275 | 0.312 a | 0.442 b | 0.367 a | 0.22 | 0.277 | 0.281 |
| Seizure-free for one year or more | 0.339 a | 0.138 | 0.181 | 0.25 | 0.13 | 0.356 a | 0.345 a | 0.153 |
Abbreviations: MoCA, Montreal cognitive assessment; HADS, Hospital Anxiety and Depression Scale; RSES, Rosenberg Self-Esteem Scale.
a P < 0.05.
b P < 0.01.
3.4. Predictive Factors of Cognitive Functions
The multivariate logistic regression analysis was used to show the predictors of the overall MoCA score. Initially, the univariate analysis showed that seizure-free (one year or more), educational level (high school and university), disease duration, and self-esteem were statistically significant at a level of P = 0.1. The multivariate logistic regression analyses showed that educational level (high school and university) and seizure-free (one year or more) were predictors of cognitive function (P < 0.05; Table 5).
| Variables | Univariate Analysis | P | Multivariate Analysis | P | ||
|---|---|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | |||
| Seizure-free for one year or more | 6.44 | 1.436 - 28.885 | 0.015 | 15.199 | 1.441 - 160.349 | 0.024 |
| Educational level (high school and university) | 7.727 | 1.416 - 42.175 | 0.018 | 16.838 | 1.364 - 207.925 | 0.028 |
| Disease duration | 0.936 | 0.866 - 1.013 | 0.1 | 0.969 | 0.888 - 1.057 | 0.477 |
| Self-esteem | 1.165 | 1.02 - 1.332 | 0.025 | 1.031 | 0.138 - 7.712 | 0.976 |
Abbreviations: OR, odds ratio; CI, confidence interval.
4. Discussion
The results of the present study revealed a high prevalence of cognitive impairment in PWTLE, with 70% of participants scoring below 26 points on the MoCA cognitive screening test (21). This finding aligns with existing research, as other studies among PWTLE have reported a prevalence of cognitive impairment ranging from 60% to 70% (22).
Further highlighting the disparity, the overage MoCA score in the current PWTLE (18.88) was significantly lower than the control group’s average (25.78). Moreover, PWTLE participants performed worse than the control group on specific subtests focusing on memory, executive function, attention, and language. These findings corroborate the literature asserting that PWTLE experience significantly more cognitive impairment than the general population and exhibit deficits across multiple cognitive domains, including language, memory, attention, visuospatial, and executive functions (3, 23).
The findings of the present study revealed that the mean MoCA score in this PWTLE sample (18.88 points) was lower than those reported in similar studies. For instance, He et al. (23) showed a MoCA score of 23.79 points in a Chinese sample, and Perez-Mojica (18) reported 24.8 points in a Puerto Rican sample (18, 23). This discrepancy might be explained, at least in part, by the sociocultural background of participants. They largely come from modest backgrounds and reside in predominantly rural regions, where research suggests that factors such as rural living and low income can negatively influence cognitive function (24).
Significantly, the results of the present study confirm that higher education level and seizure control were the main predictors of cognitive performance in PWTLE. Nevertheless, polytherapy, seizure frequency, duration of epilepsy, and low self-esteem were negatively correlated. Notably, the age of epilepsy onset, gender, depression, and anxiety did not show any significance. This finding aligns partially with the literature. Although studies support the influence of higher seizure frequency (11, 25) and longer illness duration on cognitive function (9-11, 23), Wang et al.'s study suggests that the impact of duration might be less pronounced in cross-sectional studies as ours (25).
Although the findings of the present study did not identify early age at onset as a significant factor, other studies have shown it to be one of the most powerful influences on cognitive impairment in PWTLE (10, 11, 25, 26). Regarding the effects of AEDs on cognition, the results of the present study suggest a link between polytherapy and cognitive impairment. This finding aligns with research that demonstrated a considerable negative impact of high drug load (9, 11, 26). Importantly, AEDs not only influence seizure frequency but also often affect mood and cognitive function, creating a complex and interconnected relationship (27). Moreover, it is worth noting that Vaccaro et al. identified male gender as a factor in cognitive impairment, in contrast to the findings of the current study (9).
Furthermore, the results of the current study align with existing research that suggests no direct association between cognitive and psychiatric alterations. Other studies showed no significant correlation between depressive symptoms and cognitive function levels (28). However, this finding is controversial. Nevertheless, other studies demonstrated a relevant impact of depression in TLE on both cognitive performance and overall functioning (29). Additionally, two recent studies identified anxiety as a predictor of memory functioning (14, 30).
Furthermore, the results of the current study revealed a negative correlation between advanced chronological age and visuospatial, executive, and language functions. This finding aligns with the suggestion by Griffith et al. that older adults with epilepsy might experience more rapid changes in executive functions than healthy older adults (31). However, this finding remains controversial, as a recent study demonstrated no evidence of worsening cognitive impairment in older individuals with epilepsy (32).
In addition, the results of the current study revealed a negative correlation between polytherapy and naming and memory performance. This finding partially aligns with the findings of Witt et al.’s study, which demonstrated a substantial adverse effect of higher ASM loads, particularly on executive function and memory (33). Similarly, Gimenez DeGeorge et al. observed that AED load significantly predicted poorer working memory, visuospatial memory, and auditory attention span (26). Notably, Wang et al. also reported a significant association between the number of AEDs and deficits in verbal memory, language, and psychomotor speed (25).
The impact on executive functions appears less consistent, with Agah et al.’s finding no significant association (34). The findings of the current study regarding seizure frequency also align with Wang et al.’s study, which demonstrated a significant predictive association with memory deficits (25).
The findings of the current study regarding the lack of significant association between depression, anxiety, and early age of onset with different cognitive functions diverge from those of two previous studies. Gimenez DeGeorge et al. suggested that memory impairment might be explained by earlier epilepsy onset (26). Additionally, another study reported a strong association between depression and memory impairment (32) and showed anxiety to be a predictor of memory function (35). Meanwhile, the results of the current study revealed a significant negative association between self-esteem levels and naming, attention, and language functions. Although a recent study established a connection between low self-esteem and subjective memory problems, further research is needed to solidify this emerging topic (15).
4.1. Limitations and Suggestions
The interpretation of these results requires consideration of several limitations. Firstly, the cross-sectional design and reliance on self-report measures introduce the possibility of response bias. Future studies should incorporate objective measures and longitudinal designs to strengthen the findings. Secondly, the relatively small sample size of the PWTLE group limits the potential for complex stratifications, such as by seizure type and laterality of epilepsy, which could further elucidate the observed relationships. Larger cohorts would provide richer data and enhance statistical robustness. Finally, the participant recruitment within a single region restricts the generalizability of the findings to the entire PWTLE population in Morocco. Consequently, further research with more diverse samples is crucial to validate and extend these results.
4.2. Conclusions
This study, conducted at the Day Clinic in Agadir, Morocco, revealed that low-income PWTLE experienced significantly greater cognitive impairment than healthy individuals from the same sociocultural background. The results further suggest that seizure control and higher educational level were significant predictors of cognitive performance in TLE. However, these performances were negatively correlated with low self-esteem, polytherapy, and longer disease duration. Therefore, to improve the well-being of PWTLE, the prompt diagnosis and management of comorbidities are crucial. This issue necessitates adopting comprehensive psychosocial therapeutic approaches and tailored cognitive rehabilitation programs specifically designed for the needs of TLE patients, particularly in settings with limited resources.