1. Background
Parkinson's disease (PD) is a geriatric disorder characterized by extrapyramidal predominance and nonmotor symptoms. The typical pathology involves the loss of nigrostriatal dopaminergic neurons and the accumulation of phosphorylated α-synuclein (α-syn), leading to reduced levels of dopaminergic neurotransmitters in the brain and dysfunction of the nigrostriatal motor pathway (1). The prevalence of PD increases with age, affecting approximately 1.4% of people over 60 years of age and 2.0% of those over 90. The number of patients with PD continues to rise, increasing the burdens and pressures on the state, society, and families, especially in industrialized countries (2). In 2020, there were more than 9 million PD patients worldwide, making it the second most progressive neurodegenerative disease, posing a serious threat to the physical and mental health of middle-aged and elderly people (3).
There is no complete cure for PD, so the main goal of treatment is to improve motor and nonmotor symptoms and slow disease progression (4). The primary therapeutic agents include monoamine oxidase B (MAO-B) inhibitors, dopamine agonists, and levodopa. Dopamine agonist therapy is the mainstay and is typically used in combination with MAO-B inhibitors to enhance therapeutic efficacy. Studies have shown that this combination is more effective and has a greater safety profile than its individual components (5). However, the efficacy of dopamine agonists decreases with disease progression, resulting in an overall benefit lower than that of levodopa (6). Meanwhile, the efficacy of MAO-B inhibitors is limited to short-term treatment in mild patients. Levodopa significantly controls PD symptoms but has adverse effects, including anisocoria, psychiatric disorders, and reduced efficacy over time, posing therapeutic limitations (7).
The holistic and evidence-based concepts of traditional Chinese medicine (TCM) are considered effective complements to Western medical diagnosis and treatment. Clinical evidence-based treatment based on the individual patient's condition has unique advantages in the treatment of PD. Consequently, an increasing number of academics are paying attention to TCM's characteristics and upgrading the level of TCM in diagnosing and treating PD. Some studies have shown (8, 9) that Zhengan Xifeng Decoction is a frequently used prescription for PD. It can improve patients' symptoms, inhibit disease progression, and reduce adverse effects and risks, thus compensating for the shortcomings of Western medicines. The decoction is composed of hyssop, white peony, ochre, dragon bone, oyster, tortoise board, Xuan Shen, Tian Dong, Chen Chen, neem seed, maitake, and licorice, with all herbs well mixed (10). It is widely used in neurological disorders under the guidance of heterogeneous treatment in Chinese medicine and modern pharmacological research. Research on the mechanism of action of Zhengan Xifeng Decoction on PD has progressed to the microbial and molecular levels. New research hotspots are continually being generated, focusing on the molecular effects of Zhengan Xifeng Decoction, the signaling pathways induced, and other aspects, representing the main future research directions (11).
Currently, there are no international reports on the use of Zhengan Xifeng Decoction for the treatment of PD as part of a Chinese herbal compound formula, and the present study fills this gap in the relevant field. The treatment of PD with traditional Chinese medicine is based on evidence-based therapy, and Zhengan Xifeng Decoction, a high-frequency formula, was chosen for this study because it is more meaningful than recent work in similar research areas. Moreover, this study includes all findings on Zhengan Xifeng Decoction in the treatment of PD to date, making the conclusions more representative. Due to the restricted sample size of a single study, it is challenging to provide strong support for relevant clinical efficacy assessments, which may lead to a lack of adequate understanding of the full benefits of Zhengan Xifeng Decoction in the treatment of PD. Therefore, it is necessary to conduct a meta-analysis of clinical studies and multiple outcome indicators for Zhengan Xifeng Decoction in the treatment of PD to provide a more reliable evidence-based basis for its clinical application in terms of efficacy and safety. Experimental studies have confirmed that traditional Chinese medicine has antioxidant effects and can exert anti-PD effects by regulating dopamine metabolism and iron metabolism and protecting neurons. By summarizing related studies, this meta-analysis revealed that Zhengan Xifeng Decoction combined with Western medicine for the treatment of PD not only improves clinical efficacy but also reduces the adverse effects of long-term use of Western medicine.
2. Methods
2.1. Literature Sources
CNKI, Wanfang, Weipu, PubMed, Chinese Medical Journal, China Biomedical Database, and other databases were searched. To avoid omissions and increase the rate of literature detection, both dissertations and related literature reviews were manually searched, and relevant national and international journals were consulted to ensure the most comprehensive information was obtained. For Wanfang, the following search terms were used: (“Parkinson’s disease”) and (“Zhengan Xifeng Tang” OR “Zhengan Xifeng Tang plus subtractions”) and (“Randomized” OR “Randomized Controlled” OR “Numeric Tabulation” OR “Blind” OR “RCT”) from the inception date to April 29, 2023. References of important articles were manually searched for possible relevant studies.
2.2. Literature Inclusion Criteria
- Type of study: Clinical randomized controlled trial (RCT).
- Study subject: All patients met the diagnosis of PD, and other conditions were not overly restrictive.
- Interventions: The control group used a combination of Chinese and Western medicines or Western medicines alone. Zhengan Xifeng Decoction was assessed in the experimental group, which was compared with the control group.
- Outcome indicators: Overall clinical effectiveness, total Unified PD Rating Scale (UPDRS) score or scores for Parts I-IV, and adverse events after treatment.
2.3. Literature Exclusion Criteria
- Multiple repetitions of published literature.
- Clinical controlled trials without randomized groups.
- Literature with obvious errors in statistics or methodology.
- Literature reviews, systematic evaluations, commentaries, animal experiments, clinical experiences, case reports, clinical observation reports, etc.
2.4. Data Extraction
Based on the inclusion and exclusion criteria, the literature was first screened, and relevant information was independently extracted by two researchers. Upon completion, the reviewers examined the different sections and discussed whether to include each study in cases of disagreement. If unanimity was not reached, the sections where disagreement persisted were discussed with a third-party senior reviewer to decide on inclusion. The main areas of material to be extracted are as follows:
- Basic information of the literature, including the first author, year of publication, and literary sources.
- Methods of randomized control and whether blinding was used.
- Basic information about the study subjects, such as the region where the subjects were located, the total sample size and the sample sizes of the experimental and control groups, the consistency of the condition between the two groups, the gender ratio, and the duration of the disease.
- Intervention-related information regarding the treatments given in the experimental and control groups, including the name of the drug and the course of treatment.
- Outcome-related indicators, such as the number of persons demonstrating treatment efficacy, the number of cured persons, the occurrence of adverse reactions, and other indicators related to signs and symptoms, laboratory tests, and other outcome ratings in the experimental and control groups.
2.5. Evaluation of the Quality of the Included Studies
For the methodological part of the included studies, quality assessment was completed using the Cochrane Risk of Bias Tool in RevMan 5.4. To ensure the objectivity and authenticity of the quality evaluation, two researchers first made judgments or ratings on each question or entry independently. They then discussed each question; if there were still areas of disagreement, a third-party reviewer was assigned to make a ruling on the evaluation. The key aspects assessed included random allocation methods, concealment of allocation schemes, blinding of subjects, blinding of investigators, blinding of assessors, completeness of outcome data, selective reporting of results, and bias from other sources. Depending on the literature, a choice was made between three options: High risk, unclear risk, or low risk. RevMan 5.4 software was used to generate clear and concise visualizations.
2.6. Statistical Analysis
Data from the included literature were entered into RevMan 5.4 software provided by the Cochrane Collaboration for meta-analysis. Categorical variables are expressed using the odds ratio (OR) as the statistical effect size with 95% confidence intervals (95% CIs). Continuous variables are expressed as the mean difference (MD) or standardized mean difference (SMD) with 95% CIs, depending on the situation. The I2 statistic was used to determine the degree of heterogeneity. If P was ≥ 0.1 and I2 was ≤ 50%, there was no heterogeneity between studies, and a fixed-effects model was chosen for statistical analysis. If P < 0.1 and I2 > 50%, there was significant heterogeneity among the studies, necessitating the elimination of heterogeneity by removing the results of each study one by one and then conducting sensitivity analyses to determine if it was due to a particular study. The analysis of sources of heterogeneity allows for subgroup analysis. If none of the above conditions are met and heterogeneity remains relatively high, a random effects model is used for analysis. If the number of studies was ≥ 9, a funnel plot was chosen to analyze publication bias.
3. Results
3.1. Literature Search Results
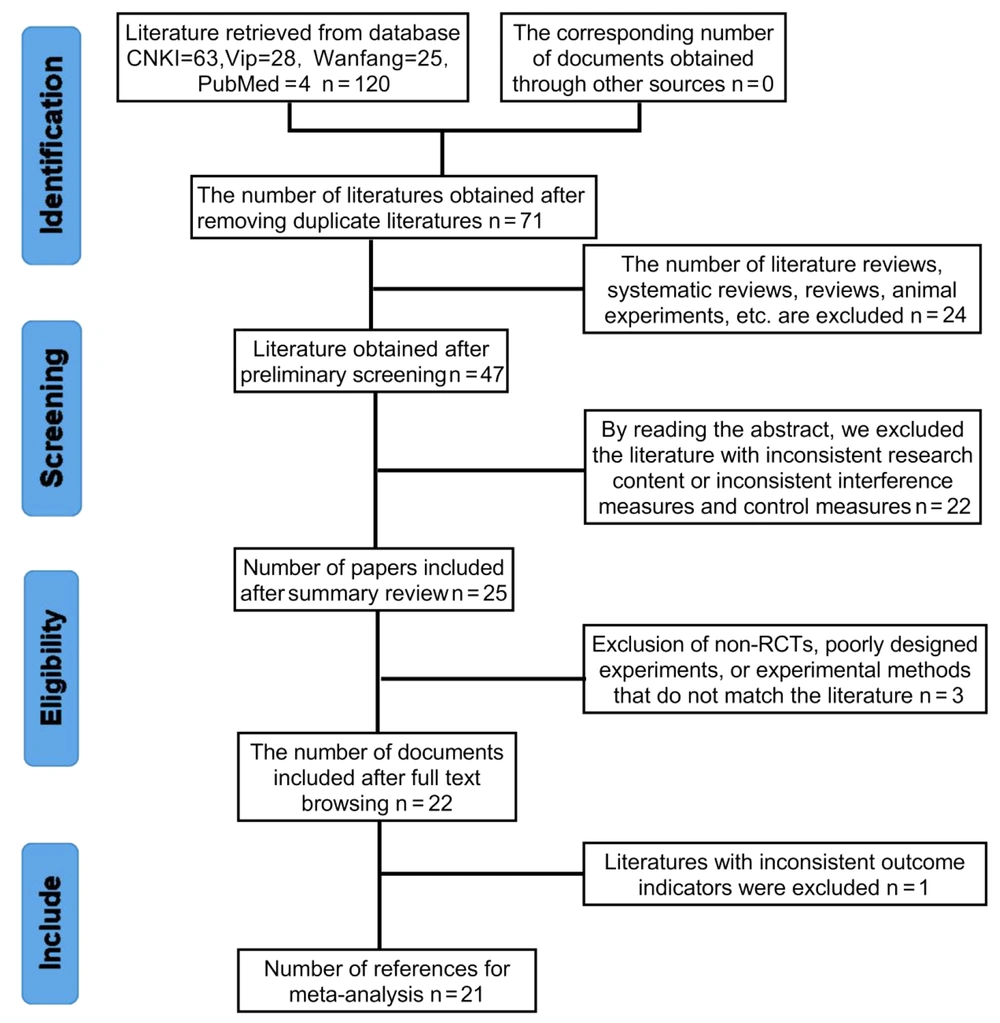
Searching the database yielded a total of 120 titles. Among them, 63 articles were obtained from the CNKI search, 25 from the Wanfang search, 28 from the Weipu search, and 4 from the PubMed search. The 120 titles were imported into EndNote software, and the number of titles after automatic rejection was 71. Then, after reading the titles of the studies, we excluded 24 documents, such as reviews, systematic evaluations, and animal studies. After reading the abstracts, we excluded 22 studies that were inconsistent with the study content, interventions, and controls. Finally, we downloaded the full texts of the remaining 25 articles and read through them to further exclude 3 articles that were mismatched with respect to nonrandomized controls, experimental design, and experimental methodology, as well as 1 article that was inconsistent in terms of the outcome metrics. Thus, we ultimately obtained 21 articles. A total of 1678 subjects were included in this meta-analysis. The specific literature screening process is shown in Figure 1.
3.2. Basic Information on the Literature Included
The final 21 papers included were all clinical randomized controlled trials with a total of 1678 patients enrolled. The control group was treated with dopa hydrazide and other conventional Western medicines, while the experimental group was treated with a combination of Zhengan Xifeng Decoction and the aforementioned medicines. The baseline levels of the included studies were comparable (Table 1).
| Inclusion of Studies | Age a (TRE/CON) | Sample Size (Cases) Treatment Group (TRE/CON) | Type of Study | Intervention (TRE/CON) | Outcome Indicator b | |
|---|---|---|---|---|---|---|
| Li (12) | 68.24 ± 9.39/68.09 ± 9.65 | 25/25 | Randomized control | Dopa hydrazine + Zhengan Xifeng Decoction | Dopa hydrazine | 1 |
| Xiaobin et al. (13) | 57.4 ± 13.1/58.8 ± 11.6 | 60/60 | Randomized control | Dopa hydrazine, Antan + ianma Hook Teng Drink + Zhengan Xifeng Decoction | Dopa hydrazine, antan | 2 |
| Jun (14) | 61.53 ± 8.08 | 38/38 | Random number | Dopa hydrazine + Zhengan Xifeng decoction | Dopa hydrazine | 4 |
| Yingfei and Xu (15) | 65.42 ± 12.68/64.19 ± 12.05 | 21/21 | Randomized control | Dopa hydrazine + Zhengan Xifeng Decoction | Dopa hydrazine | 3 |
| Jinhai (16) | 60.4 ± 7.8 | 31/31 | Randomized control | Dopa hydrazine + Zhengan Xifeng Decoction | Dopa hydrazine | 4 |
| Yu and Aiyun (17) | 60.5 ± 12.2/60.8 ± 13.1 | 28/28 | Randomized control | Dopa hydrazine + Zhengan Xifeng Decoction | Dopa hydrazine | 1 |
| Huaxing et al. (18) | 70.29 ± 7.64/71.23 ± 7.62 | 40/40 | Two-color allocation | Dopa hydrazine + Zhengan Xifeng Decoction | Dopa hydrazine | 4 |
| Wenjie et al. (19) | 60.9 ± 9.1/60.4 ± 8.7 | 41/41 | Random number | Dopa hydrazine, donepezil hydrochloride tablets + Zhengan Xifeng Decoction | Dopa hydrazine, donepezil hydrochloride tablets | 4 |
| Qinwu (20) | 68.25 ± 9.40/68.26 ± 9.41 | 50/50 | Randomization method | Dopa hydrazine + Zhengan Xifeng Decoction | Dopa hydrazine | 3 |
| Li and Qingju (21) | 54.79 ± 5.72/63.25 ± 5.12 | 30/30 | Random number | Dopa hydrazine + Zhengan Xifeng Decoction | Dopa hydrazine | 3 |
| Xianwen (22) | 63.2 ± 10.6/64.1 ± 11.0 | 45/45 | Randomization method | Dopa hydrazine + Zhengan Xifeng Decoction | Dopa hydrazine | 3 |
| Lu et al. (23) | 62.87 ± 5.58/63.57 ± 5.42 | 47/47 | Random number | Dopa hydrazine + Zhengan Xifeng Decoction | Dopa hydrazine | 4 |
| Wenlan et al. (24) | 60.23 ± 10.21/61.25 ± 9.82 | 68/68 | Digital tabulation | Dopa hydrazine + Zhengan Xifeng decoction | Dopa hydrazine | 4 |
| Kexian et al. (25) | 68.14 ± 7.34/68.09 ± 7.33 | 36/36 | Random number | Dopa hydrazine, metronidazole + Zhengan Xifeng Decoction combined with Huanglian and Agaricusiae Soup | Dopa hydrazine, metronidazole | 4 |
| Yunyun (26) | 72.17 ± 4.63/73.65 ± 4.21 | 47/47 | Random number | Dopa hydrazine + Zhengan Xifeng Decoction | Dopa hydrazine | 4 |
| Luoxi et al. (27) | 51.61 ± 5.96/50.16 ± 5.87 | 46/46 | Random number | Dopa hydrazine + Zhengan Xifeng Decoction | Dopa hydrazine | 3 |
| Zhenzhang and Hanxiang (28) | 68.43 ± 7.65/69.23 ± 6.78 | 34/34 | Randomized control | Dopa hydrazine + Zhengan Xifeng Decoction | Dopa hydrazine | 1 |
| Keke and Yunzhi (29) | 60.93 ± 7.77/61.82 ± 7.35 | 28/28 | Randomized control | Dopa hydrazine + Zhengan Xifeng Decoction | Dopa hydrazine | 4 |
| Liping (30) | 61.90 ± 6.80/60.43 ± 8.09 | 28/28 | Randomized control | Dopa hydrazine + Zhengan Xifeng Decoction | Dopa hydrazine | 4 |
| Xin and Panpan (31) | 65.94 ± 13.41/66.45 ± 13.29 | 50/50 | Random number | Dopa hydrazine + Zhengan Xifeng Decoction | Dopa hydrazine | 3 |
| Lingfei (32) | 65.87 ± 6.17/64.80 ± 5.88 | 46/46 | Random number | Dopa hydrazine + Zhengan Xifeng Decoction | Dopa hydrazine | 3 |
Abbreviations: TRE, treatment group; CON, control group.
a Values are expressed as mean ± standard deviation.
b 1: UPDRS I, II, and III scores; 2: UPDRS II and III scores; 3: overall UPDRS score; 4: efficacy.
3.3. Risk of Bias Assessment
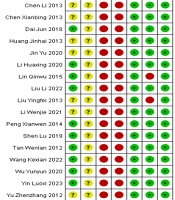
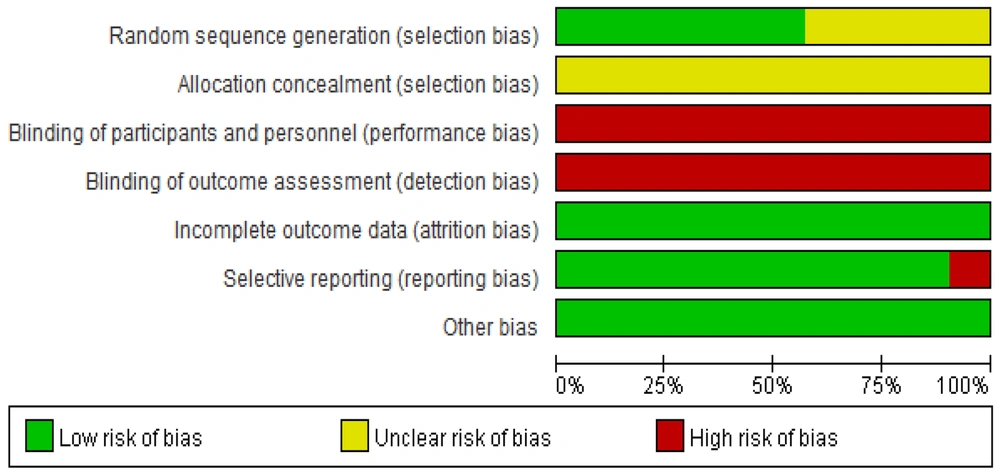
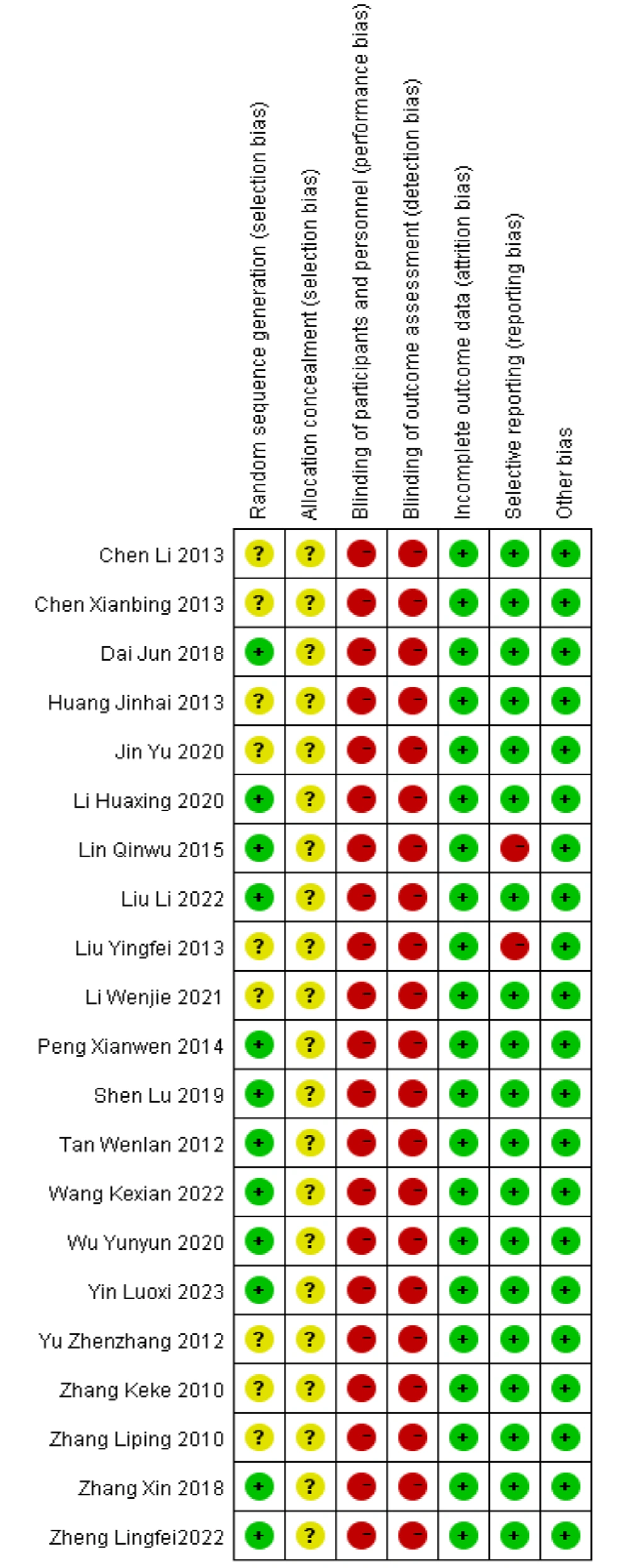
The results of the risk of bias assessment of the included studies are shown in Figures 2. , 3. Assessment methods were recommended by the International Cochrane Collaboration. The included studies were all clinical randomized controlled trials. In 8 studies (14, 21, 23-25, 27, 31, 32), patients were randomly assigned using the randomized numeric table method. In 1 study (18), patients were assigned using the two-color ball method, and in 3 studies (20, 22, 26), patients were assigned using the randomized lottery method. These studies were evaluated as “low risk” for bias. The remaining 9 studies (12-17, 19, 28-30) were evaluated as “unclear” because they only mentioned "randomized grouping". None of the 21 papers described allocation concealment, so this factor was rated as “unclear”. The blinding of participants, testers, and outcome assessments was not mentioned in any of the studies, so this factor was assessed as “unclear”. The first two studies were evaluated as “high risk”, and the blinded treatment for outcome evaluation was evaluated as “unclear”.
In terms of data completeness, none of the 21 included papers had missing data, and the outcomes were reported completely. Thus, these studies were assessed as “low risk”. Two papers (15, 20) did not fully report the predefined outcome indicators and were rated as “high risk”, whereas the remaining 19 papers reported the predefined outcome indicators and were therefore rated as “low risk”. No other bias was found in any of the 21 included papers, and it was uncertain whether any other bias existed. Thus, these papers were considered “low risk”.
3.4. Meta-analysis Results
Of the 21 included papers, the treatment regimen was Zhengan Xifeng Decoction combined with conventional Western medicines. All the studies reported clinical efficacy, with 7 of them (15, 20-22, 27, 31, 32) reporting total UPDRS scores for Zhengan Xifeng Decoction combined with dopa hydrazide compared to dopa hydrazide alone. Three of the papers (12, 17, 28) used UPDRS I, II, and III scores as outcome indicators; one (13) used UPDRS II+III scores; one (19) used UPDRS I, II, III, and IV scores; and another (32) used UPDRS II and III scores as well as total scores. The remaining papers did not mention UPDRS scores.
3.5. Forest Plot of the Meta-analysis of Total Efficacy in the Two Groups of Parkinson's Disease Patients
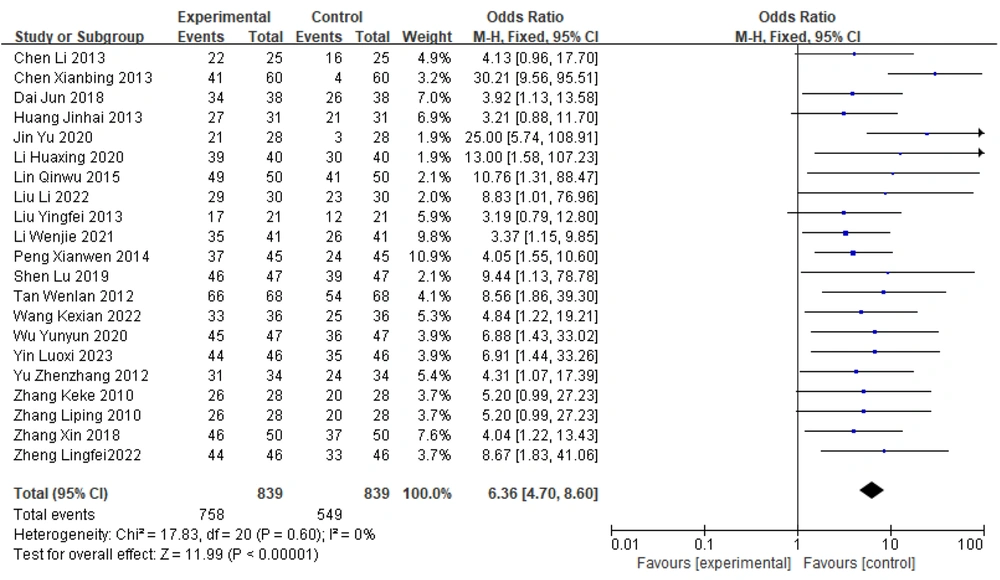
Among the 21 papers included in the present study, all reported differences in the overall effectiveness of Zhengan Xifeng Decoction and the control group in treating patients with PD. There was no heterogeneity between the results of multiple studies [χ2 = 17.83, df = 20 (P = 0.60); I2 = 0%]. Statistical analyses were performed using a fixed-effects model, and the results of the combined effect sizes [OR = 6.36, 95% CI (4.70, 8.60), Z = 11.99 (P < 0.00001)] indicate a statistically significant difference between the two groups. These findings indicate that for patients with PD, the therapeutic efficacy of Zhengan Xifeng Decoction combined with Western medicine was greater than that of the control group, as shown in Figure 4.
3.6. Comparison of UPDRS Scores Between the Two Groups of Parkinson's Disease Patients
3.6.1. Overall UPDRS Score
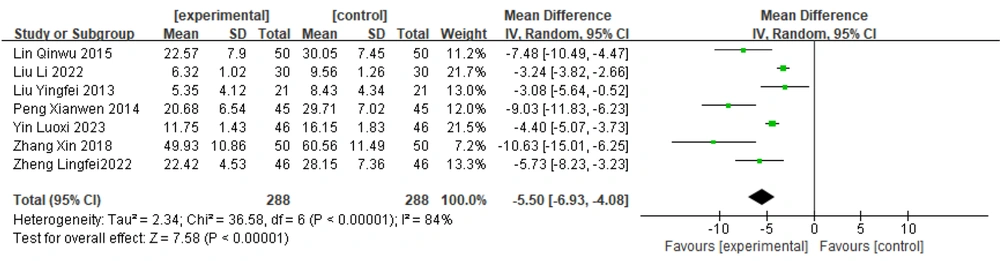
Seven papers (15, 20-22, 27, 31, 32) reported total UPDRS scores for Zhengan Xifeng Decoction combined with dopa hydrazide tablets. Heterogeneity tests were performed on these studies, and the results showed substantial heterogeneity (P < 0.00001, I2 = 84%). Therefore, a random effects model was chosen to combine the effect sizes. The results [MD = -5.50, 95% CI (-6.93, -4.08)] suggested statistically significant differences between groups [Z = 7.58 (P < 0.00001)]. These findings indicate that, based on improved UPDRS scores, the efficacy of Zhengan Xifeng Decoction combined with conventional Western drugs is more significant than that of Western drug treatment alone. The details are presented in Figure 5. Sensitivity analyses were also performed to determine the causes of heterogeneity.
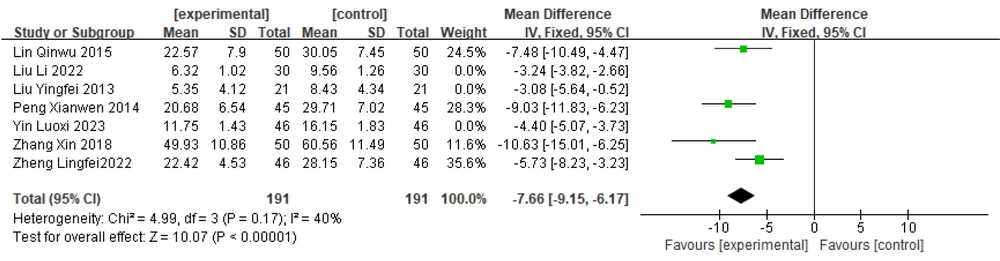
Sensitivity analysis: Sensitivity analysis of this group revealed no heterogeneity among the four studies (20, 22, 31, 32) (P = 0.17, I2 = 40%). Therefore, a fixed-effects model was chosen to combine the effect sizes: [MD = -7.66, 95% CI (-9.15, -6.17)]; [Z = 10.07, P < 0.00001]. The results revealed that the difference between the groups was statistically significant, indicating that the effect of treatment with Zhengan Xifeng Decoction combined with conventional Western medicine on reducing the UPDRS score was greater than that of treatment with Western medicine alone. The details are presented in Figure 6.
There was also no heterogeneity among the remaining three studies (15, 27) (P = 0.33, I2 = 0%). Therefore, a fixed-effects model was chosen to combine the effect sizes ([MD = -4.32, 95% CI (-4.96, -3.67)], [Z = 13.03, P < 0.00001]). Similarly, there was no heterogeneity between the other two studies (15, 21) (P = 0.90, I2 = 0%). Therefore, a fixed-effects model was chosen to combine the effect sizes ([MD = -3.23, 95% CI (-3.80, -2.67)], [Z = 11.20, P < 0.00001]). The results showed statistically significant differences between the groups. The results of both subgroups suggest that in the treatment of PD, the efficacy of Zhengan Xifeng Decoction in combination with dopa hydrazine is superior to that of Western medicine alone.
3.6.2. UPDRS I+II+III Scores
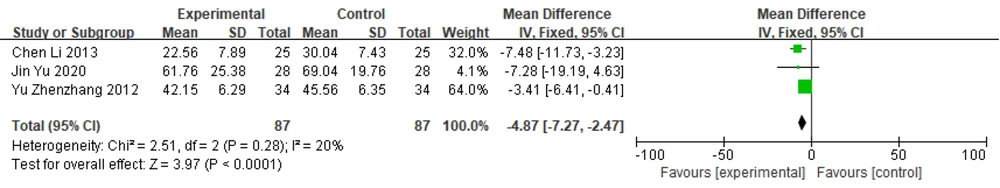
Three studies (12, 17, 28) used the UPDRS I+II+III total score as an outcome indicator. A heterogeneity test was performed and revealed no heterogeneity among these studies (P = 0.28, I2 = 20%). Therefore, a fixed-effects model was chosen for merging the effect sizes [MD = -4.87, 95% CI (-7.27, -2.47)]. The results suggested a statistically significant difference between the groups [Z = 3.97, P < 0.0001]. These findings suggest that in the treatment of Parkinson's disease, the efficacy of Zhengan Xifeng Decoction combined with dopa hydrazine is superior to that of Western medicine alone (Figure 7).
3.6.3. UPDRS II and III Scores
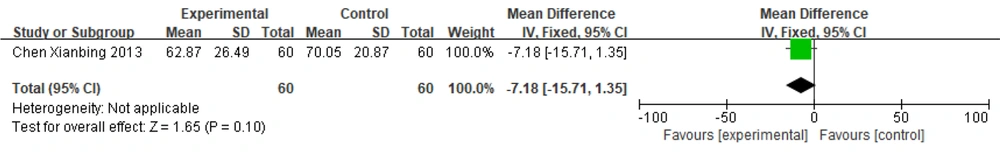
One paper (13) used the UPDRS II and III scores as outcome indicators and used a fixed-effects model to combine effect sizes: [MD = -7.18, 95% CI (-15.71, 1.35)], Z = 1.65 (P = 0.10). The results showed no statistically significant difference between the two groups. This indicates that there was no difference in the effect between the experimental group treated with Zhengan Xifeng Decoction + Tian Ma Gou Teng Yin combined with Western medicine and the control group treated with conventional Western medicine alone in terms of reducing UPDRS II and III scores (Figure 8).
3.6.4. UPDRS I Score
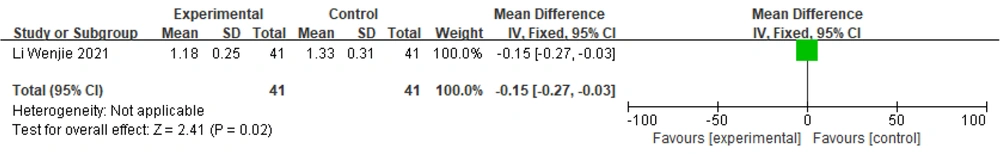
One study (19) reported UPDRS I scores, and a fixed-effects model was chosen to combine effect sizes: [MD = -0.15, 95% CI (-0.27, -0.03)], [Z = 2.41, P = 0.02]. The results showed a statistically significant difference between the groups, indicating that the therapeutic effect of Zhengan Xifeng Decoction combined with conventional Western medicines was better than that of conventional Western medicines alone (Figure 9).
3.6.5. UPDRS II Score
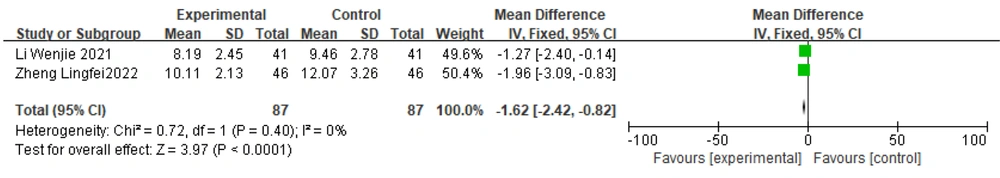
Two studies (19, 32) reported UPDRS II scores. The test for heterogeneity indicated no heterogeneity between the two studies (P = 0.40, I2 = 0%). Therefore, a fixed-effects model was chosen to combine effect sizes, and the results suggested statistically significant differences between the groups. The standardized mean difference with 95% confidence intervals was [-1.62 (-2.42, -0.82)]. These results suggest that in the treatment of PD, the efficacy of Zhengan Xifeng Decoction combined with Western medicine is better than that of Western medicine alone (Figure 10).
3.6.6. UPDRS III Score
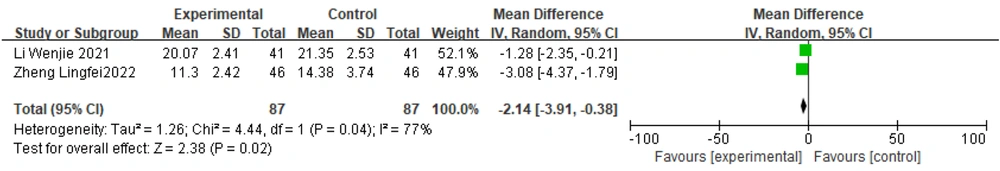
Two studies (19, 32) reported UPDRS III scores, and a heterogeneity test revealed considerable heterogeneity between them (P = 0.04, I2 = 77%). Therefore, a random effects model was chosen to combine the effect sizes. The results showed a statistically significant difference between the two groups [Z = 2.38, P = 0.02]. The standardized mean difference with 95% confidence intervals was [-2.14 (-3.91, -0.38)]. These results suggest that in the treatment of PD, the efficacy of Zhengan Xifeng Decoction combined with Western drugs is better than that of Western drugs alone (Figure 11).
3.6.7. UPDRS IV Score
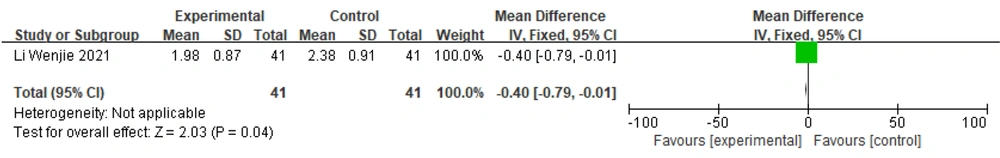
One study (19) reported UPDRS IV scores; therefore, a fixed-effects model was chosen to combine effect sizes: [MD = -0.40, 95% CI (-0.79, -0.01)], [Z = 2.03, P = 0.04]. The results suggested that the difference between the groups was statistically significant. This indicates that the effect of Zhengan Xifeng Decoction combined with conventional Western medicine in the treatment of PD is better than that of conventional Western medicine alone (Figure 12).
3.7. Adverse Reactions
Ten of the 21 papers reported records related to adverse reactions, whereas the remaining 11 papers did not contain any reports of adverse reactions. Two of them (15, 32) reported no adverse events in either the control or experimental group, and one (22) reported no adverse reactions. Symptoms related to adverse reactions, such as nausea, vomiting, decreased appetite, insomnia, constipation, and dizziness, were mentioned in four studies (13, 21, 23, 31). In these studies, the number of adverse reactions in the test group was 30, while the control group had 43. One study (27) mentioned adverse reactions in terms of liver function, renal function, gastrointestinal tract function, cardiac arrhythmia, and mental mood. The incidence of adverse reactions in the experimental group was 13.04% (6/46), and in the control group, it was 17.39% (8/46). The comparison between the two groups suggested that the difference was not statistically significant (P > 0.05). One study (28) reported that, according to the evaluation of the UPDRS 3.0 IV toxic side effect scale score, the combination of Zhengan Xifeng Decoction and Western medicines in the treatment of PD not only reduces the toxic side effects of dopa hydrazine but also improves the symptoms of UPDRS IV in patients compared to Western medicine therapy alone. Another study (24) reported that before and after treatment, adverse reactions were categorized as none, mild, moderate, or severe, and were scored as 0, 1, 2, or 3, respectively, according to the severity of different signs and symptoms. The average toxicity and adverse reaction scores before and after treatment were compared between the treatment group and the control group, and the scores in both groups were significantly lower (P < 0.05). The occurrence of specific adverse reactions is shown in Table 2.
| Inclusion of Literature | Adverse Reaction (Experimental Group/Control Subjects) |
|---|---|
| Li (12) | Not mentioned/not mentioned |
| Xiaobin et al. (13) | Nausea and vomiting (2 cases), poor appetite (5 cases), insomnia (8 cases), constipation (3 cases)/nausea and vomiting (1 case), poor appetite (12 cases), thirst (5 cases), constipation (15 cases) |
| Jun (14) | Not mentioned/not mentioned |
| Yingfei and Xu (15) | None/none |
| Jinhai (16) | Not mentioned/not mentioned |
| Yu and Aiyun (17) | Not mentioned/not mentioned |
| Huaxing et al. (18) | Not mentioned/not mentioned |
| Wenjie et al. (19) | Not mentioned/not mentioned |
| Qinwu (20) | Not mentioned/not mentioned |
| Li and Qingju (21) | Nausea and vomiting (1 case), rash (1 case)/nausea and vomiting (1 case), diarrhea (1 case), rash (1 case) |
| Xianwen (22) | None/none |
| Lu et al. (23) | Anorexia (1 case), nausea (3 cases), constipation (3 cases)/anorexia (1 case), nausea (2 cases), constipation (2 cases) |
| Wenlan et al. (24) | Specific data not clear/specific data not clear |
| Kexian et al. (25) | Not mentioned/not mentioned |
| Yunyun (26) | Not mentioned/not mentioned |
| Luoxi et al. (27) | Specific data not clear/specific data not clear |
| Zhenzhang and Hanxiang (28) | Specific data not clear/specific data not clear |
| Keke and Yunzhi (29) | Not mentioned/not mentioned |
| Liping (30) | Not mentioned/not mentioned |
| Xin and Panpan (31) | Dizziness (1 cases), nausea (1 case), insomnia (1 case)/dizziness (1 case), nausea (1 case) |
| Lingfei (32) | None/none |
3.8. Publication Bias Test
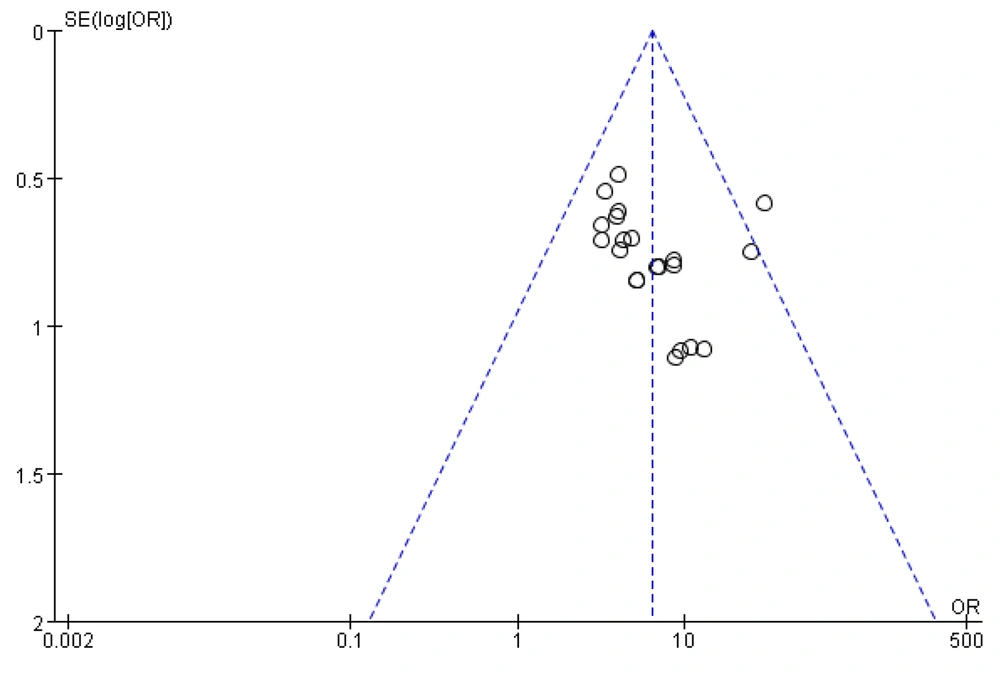
To assess publication bias in the literature, an inverted funnel plot was drawn using RevMan 5.4 software, with the efficiency rate used as an indicator. The horizontal coordinate represents the effect size (OR value), and the vertical coordinate represents the standard error (SE value). Publication bias was determined by observing whether the small circles representing the individual studies in the funnel plot were evenly and symmetrically distributed on either side of the midline.
As shown in the figure, a large number of studies are concentrated above the inverted funnel plot, and the graphical distribution is asymmetrical from left to right, with one study distributed outside the inverted funnel plot and one at the edge. This finding suggests the presence of publication bias or low methodological quality. The reasons for this could include issues such as blinding, imprecise implementation of allocation concealment, and small sample sizes in the included trials, possibly associated with the inclusion of poorer-quality studies (Figure 13).
4. Conclusions
There is no specific term for Parkinson's disease in Chinese medicine, but its clinical manifestations can be attributed to categories such as "tremor disease" and "tremor evidence." As early as in the "Yellow Emperor's Classic of Internal Medicine," it is discussed as part of the "disease shaking" records. Symptoms like shaking and dizziness are considered indicative of PD movement disorders related to liver issues. It is believed that the internal movement of liver wind, the loss of tendon and vein nourishment, or the onset of disease due to kidney water deficiency, which fails to nourish liver wood, are key factors (33). The pathological manifestation is a combination of deficiency and excess. Specifically, the weakness of qi, blood, yin, and yang is the underlying cause, while wind, fire, phlegm, and stasis are the pathological hallmarks. The deficiency of liver and kidney yin and the internal movement of liver wind are the main etiology and pathogenesis of PD (34).
Zhengan Xifeng Decoction is safe and effective, improving the clinical symptoms of PD patients at multiple target levels. The hyssop in this formula promotes blood circulation, removes blood stasis, nourishes the liver, and tonifies the kidney. Ochre calms the liver, submerges yang, and subdues rebelliousness. Dragon’s bone calms the heart and mind and subdues yang. Oyster also calms the liver, submerges yang, and is astringent. White peony nourishes liver blood, suppresses liver yang, softens tendons, relieves pain, and has the effect of tonifying qi, nourishing blood, and improving eyesight. Turtle board nourishes the heart, tranquilizes the mind, benefits yin, and submerges yang. Xuan Shen and Tian Dong nourish the kidney and yin. Raw Mal moves qi and eliminates stagnation. Yin Chen relieves bile and yellow, clearing heat and dampness. Neem Zi clears and drains liver yang. The combination of all these medicines can nourish the liver and kidney, calm the liver, quench the wind, and achieve therapeutic effects.
Currently, the main cause of dopamine neurodegeneration in PD patients is unclear. It is speculated that genetic and environmental factors lead to abnormal mitochondrial accumulation, causing oxidative stress, increased toxic burden, and ultimately triggering the death of dopaminergic neurons (35). Long-term use of Western medications for PD can cause various side effects, making herbal medicine a popular alternative therapy (36). The literature suggests that Zhengan Xifeng Decoction positively affects the abnormal degenerative loss of DA neurons, malfunctions in the synthesis of neurotrophic factors, oxidative stress, and abnormal expression of proteolytic enzymes in PD patients. Therefore, it is hypothesized that Zhengan Xifeng Decoction can inhibit the development of PD by preventing DA neuron apoptosis, regulating DA neuron-related proteases, modulating peroxidation, adjusting neurotrophic factor content, and downregulating α-synuclein proteins.
Modern pharmacological experiments have shown that cow’s knee helps maintain α-synuclein homeostasis and promote neuronal cell growth (37). Ochre acts predominantly as an anti-inflammatory agent, sedative, and anticonvulsant (38). White peony extract reduces MPTP induction in mice and protects DA neuronal cells by modulating neuroinflammatory factors (39). Tortoise board can increase dopamine levels in the striatum of PD model animals and play a neuroprotective role by upregulating nerve growth factor (NGF) expression and inhibiting cell apoptosis (40-42). The chemical constituents in Gentiana scabra are neuroprotective in a desmoplastic model of neurotrophic factor deprivation injury and a fisetinone model of specific injury to nigrostriatal DA neurons (43). Neem extracts can inhibit microglia-mediated inflammation (44). The main constituent of asparagus, alcohol-sinking polysaccharides, can inhibit oxidative stress and immune responses, reducing neuronal degeneration through the stimulation of tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), and interleukin-6 (IL-6) cytokines (45). Licorice inhibits the metabolism of lipid peroxides. Inulin has been shown to have favorable effects on nervous system regulation and protection (46). Malt fiber, the active ingredient in malt, can reduce the misfolding of α-synuclein by improving intestinal flora and preventing excessive nigrostriatal DA neuronal deficits (47). Lobelia and oyster contain a variety of trace elements, and their extracts have an inhibitory effect on oxidative stress.
Some studies have shown that most herbal compound preparations and their extracts have antioxidant effects and can protect neurons and exert anti-PD effects by regulating dopamine and iron metabolism (48, 49). Another meta-analysis indicated that Chinese herbal medicine, when used as an adjuvant drug for PD, is superior to Western drugs alone. It significantly improves UPDRS scores, particularly motor and nonmotor symptoms of PD, and reduces the adverse effects of Western drugs, making it suitable for long-term use in PD patients (50, 51).
In summary, according to both traditional Chinese medicine and modern pharmacology, the individual components of Zhengan Xifeng Decoction have a protective effect on DA neurons and thus play a role in the treatment of PD. Therefore, this meta-analysis was performed to comprehensively evaluate the therapeutic efficacy of Zhengan Xifeng Decoction.
A total of 21 papers were included in this meta-analysis and were grouped according to different outcome indicators. Subgroup analyses were performed based on different interventions. The results suggested that in the basic treatment of PD, the combination of Zhengan Xifeng Decoction with conventional Western medicine was superior to Western medicine alone in terms of increasing the overall effective rate and decreasing the UPDRS score. In the subgroup of Zhengan Xifeng Decoction combined with dopa hydrazide vs. dopa hydrazide alone, there was a high degree of heterogeneity among the studies in terms of the total UPDRS score. This heterogeneity may be attributed to the following factors: (1) the literature spans a long period; (2) the age and disease duration of the patients at baseline were different; (3) the treatment course varied; and (4) the administered dose was not uniform.
Taken together, these findings indicate that Zhengan Xifeng Decoction combined with Western medicine has better prospects than conventional Western medicine alone. Additionally, when Zhengan Xifeng Decoction was used for the treatment of PD, the incidence of side effects decreased, and the clinical use of the drug was relatively safe.
The 21 articles included in this study were of low quality. Methodologically, the following points should be noted: (1) there may have been pseudorandomization in the included studies; (2) various aspects of the included trials, such as concealment of random allocation methods, blinding, reporting of research results, and other biases, were not specifically described; (3) the subjects included in this study may have had different disease severities and treatment effects; (4) the funnel plot of this study lacked symmetry, indicating publication bias, and the inclusion of research literature may have led to incorrect data; (5) fewer studies reported safety indicators, making further safety analysis impossible.
In terms of diagnostic criteria, the diagnostic criteria developed by the British Brain Bank are mostly adopted internationally. In this study, we used the diagnostic criteria for PD developed by the 1984 National Symposium on Extrapyramidal Diseases, the 2016 edition of the Clinical Diagnostic Criteria for PD developed by the Chinese Medical Association and its subcommittee, and the diagnostic criteria for neurology in its 6th edition and 2008 edition. The outcome metrics were scored using the UPDRS. For studies that did not report UPDRS scores, efficiency was determined based on the WEBSTER and PDQ-39 scores, calculated using the nimodipine method. An improvement of 20% or greater after treatment was considered effective, whereas an improvement of less than 20% was considered ineffective.
4.1. Limitations
This study has the following limitations: (1) the included studies were from China and of low quality, with deficiencies in the methodological design, thus reducing the credibility of the conclusions; (2) there is a possibility of underdetection; (3) there are differences in baseline balance, interventions, diagnostic criteria, and outcome indicators.
Due to the limitations of the experimental conditions, only trials published in Chinese were included in our study, and all the included studies were conducted in China, which potentially affects the generalizability of the results. Therefore, it is necessary to seek international cooperation to obtain a wider range of experimental data in future studies. Moreover, the quality of the included studies was low, and none of the studies involved randomized double-blind implementation, which reduces the reliability of the experimental results. Some of the included studies did not report UPDRS scores and only reported treatment efficacy rates, resulting in differences in outcome metrics and a reduced level of evidence. The diagnostic criteria and pharmacological interventions of the included studies were not standardized, which may have led to differences in treatment effectiveness and UPDRS scores. Additionally, incomplete safety reports may impact the study results, subsequently affecting the authenticity and reliability of the systematic evaluation.
4.2. Conclusions
Western medicine has more experience and a more standardized scientific treatment plan for PD. According to modern medicine, the preferred treatment for PD is extracorporeal dopamine supplementation or the use of dopamine analogs. With continuous progress in research on drug-targeted therapy for PD, there are corresponding types of Western drugs that address the patient's age, clinical symptoms, and duration of the disease, making Western drug therapy clinically preferred. Western medical treatment for PD includes surgical treatment, cell transplantation, and gene therapy, in addition to medication, which effectively increases dopamine levels in the body and improves the patient's motor symptoms (52, 53).
Chinese medicine is often perceived as less effective than Western medicine for treating diseases, particularly chronic diseases like PD. Many people subjectively and wrongly believe that Chinese medicine cannot cure such diseases. However, for PD patients who need lifelong medication, Chinese medicine can reduce the amount of Western medication required and mitigate the adverse effects of long-term Western medication use. Combining Chinese and Western medicine can provide more effective treatments for PD.
In recent years, the number of clinical trials applying Zhengan Xifeng Decoction for the treatment of PD has been increasing. This study is a comprehensive analysis of the results from many studies on the combination of Zhengan Xifeng Decoction with Western medicines for PD treatment. The current results show that this combination can increase the overall rate of clinical efficacy, improve the UPDRS score, and enhance the safety profile. However, considering the low quality of the included studies, future randomized clinical trials (RCTs) need to pay more attention to objective science, improve sample quality, rigorously verify the clinical efficacy and safety of the drug, and perform long-term follow-up to observe the treatment's efficacy and adverse effects in PD patients.