1. Background
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has been the cause of COVID-19 and has presented significant challenges since the early stages of the outbreak (1). Current evidence suggests that older adults and individuals with underlying comorbidities, such as cardiovascular disease, diabetes, chronic lung diseases, and neurological disorders, may be more prone to severe outcomes from COVID-19 (2-4).
Parkinson’s disease (PD), a prevalent neurodegenerative disorder of the central nervous system, is more commonly diagnosed in older adults and is often accompanied by other comorbid conditions (5). Given that COVID-19 tends to result in more adverse outcomes among patients with chronic diseases and in the elderly, there is a concern that PD patients may face a higher risk of contracting COVID-19 (6). Recent research indicates that COVID-19 may independently trigger the onset of Parkinson’s disease, highlighting the critical importance of exploring the interaction between COVID-19 and Parkinson’s disease (7, 8).
As the coronavirus pandemic persists, an increasing body of observational studies has been documenting the clinical characteristics and outcomes of COVID-19 in PD patients. Yet, a definitive consensus on the effects of COVID-19 infection on individuals with Parkinson’s disease remains elusive.
2. Objectives
This study was undertaken to investigate the prevalence, clinical features, and outcomes of COVID-19 among Iranian patients with PD.
3. Methods
3.1. Study Design and Population
This cross-sectional study was conducted at Alzahra and Kashani hospitals, which are affiliated with Isfahan University of Medical Sciences in Isfahan, Iran, from July to September 2021. These hospitals serve the largest population of PD patients in Isfahan and the neighboring regions. All patients who had been diagnosed with PD by a neurologist and were registered at these hospitals were eligible for inclusion in the study.
3.2. Data Collection
Participants in the study were individuals with a confirmed diagnosis of Parkinson's disease by specialist neurologists in accordance with the established inclusion criteria. Contact was made via the telephone numbers provided or through their immediate family members. Cases, where there was no response, unwillingness to participate, or incorrect contact information, were noted as non-participation.
Patients were requested to respond to a structured three-part checklist over the phone. Part 1 collected demographic data such as age, gender, marital status, weight, and height. Part 2 inquired about disease specifics, including smoking habits, duration of illness, comorbid conditions, and medication use. Part 3 involved questions about the presence of COVID-19 symptoms (e.g., fever, cough, shortness of breath, fatigue, muscle pain, dizziness, and weakness) and diagnoses among their family members. Further, patients were asked if they had experienced COVID-19 symptoms in recent months, undergone COVID-19 testing, been hospitalized for COVID-19, or suffered any post-COVID-19 complications. In the event of a patient's death, family members were contacted to determine whether COVID-19 was a contributing cause.
3.3. Ethics
The bioethics committee of Isfahan University of Medical Sciences granted approval for this study (IR.MUI.MED.REC.1400.113). Informed consent was obtained from all participating patients. For interviews conducted by telephone, verbal consent was secured before the commencement of the checklist inquiries.
3.4. Statistical Analysis
Descriptive data are presented as mean (standard deviation) for continuous variables and as frequency (percentage) for categorical variables. A logistic regression model was used to assess the impact of demographics (age, sex, marital status, BMI, smoking, and blood group) and clinical characteristics (age at onset, disease duration, medication use, comorbidities, and the use of vitamin D, aspirin, and either Ibuprofen or Gelofen) on COVID-19 outcomes. Initially, a univariate model identified the influence of each independent variable on COVID-19 infection. Subsequently, risk factors associated with the univariate analysis were incorporated into a multivariate model. A backward selection process was then employed to identify the most significant factors related to COVID-19 in the final multivariate model. The outcomes of the logistic regression analyses are reported as odds ratios (OR), 95% confidence intervals (CI), and P-values. A significance level was established at 0.05 (2-tailed). All statistical analyses were conducted using IBM SPSS Statistics (version 18; IBM Corporation, Armonk, NY, USA).
4. Results
4.1. Demographic Characteristics
Of the 567 PD patients contacted, 558 responded and participated in the study. The average age was 65.14 (± 12.26) years, with a majority being male (62.6%). The mean age at onset and disease duration were 58.46 (± 13.04) years and 6.71 (± 5.20) years, respectively. Levodopa (78.3%) and Amantadine (54.8%) were the most frequently used medications. Hypertension (HTN), cardiovascular disease (CVD), and diabetes mellitus (DM) were the most prevalent comorbidities, affecting 36.8%, 21%, and 20.6% of the patients, respectively. The demographic and clinical characteristics of the patients are summarized in Table 1.
| Variables | Values |
|---|---|
| Age | 65.14 ± 12.26 |
| Sex | |
| Male | 355 (62.6) |
| Female | 212 (37.4) |
| Marital status | |
| Single | 53 (9.3) |
| Married | 514 (90.7) |
| BMI | 26.02 ± 4.33 |
| Smoking | |
| Yes | 46 (8.1) |
| No | 520 (91.9) |
| Age at onset | 58.46 ± 13.04 |
| Disease duration | 6.71 ± 5.20 |
| Who takes care of you | |
| Myself | 285 (50.4) |
| Nurse | 13 (2.3) |
| My family | 268 (47.3) |
| Drug | |
| Levodopa | 445 (78.3) |
| Amantadine | 311 (54.8) |
| Pramipexole | 177 (31.2) |
| Biperiden | 40 (7) |
| Selegiline | 12 (2.1) |
| Trihexyphenidyl | 92 (16.2) |
| Vitamin D supplement | |
| Yes | 327 (58) |
| No | 237 (42) |
| Comorbidity | |
| CVD | 119 (21) |
| DM | 117 (20.6) |
| Asthma | 14 (2.5) |
| Lung | 44 (7.7) |
| HTN | 209 (36.8) |
| Cancer | 9 (1.6) |
| Stroke | 46 (8.1) |
| Kidney | 58 (10.2) |
| Liver | 13 (2.3) |
| No comorbidity | 233 (100) |
| Aspirin | |
| Yes | 194 (34.3) |
| No | 371 (65.7) |
| Ibuprofen or Gelofen | |
| Yes | 142 (25.1) |
| No | 424 (74.9) |
a Values are presented as mean ± SD or No. (%).
4.2. History of COVID-19 Among Patients' Family Members
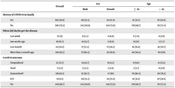
The prevalence of COVID-19 among patients’ families was 29.8%; of these, 13.7% were hospitalized, and 4.2% died (Table 2).
| Overall | Sex | Age | |||
|---|---|---|---|---|---|
| Male | Female | ≤ 65 | > 65 | ||
| History of COVID-19 in family | |||||
| Yes | 168 (29.8) | 110 (31.3) | 58 (27.4) | 81 (31.3) | 87 (28.6) |
| No | 396 (70.2) | 242 (68.8) | 154 (72.6) | 178 (68.7) | 217 (71.4) |
| When did she/he get the disease | |||||
| Last week | 10 (6) | 6 (5.5) | 4 (6.9) | 6 (7.4) | 4 (4.6) |
| Two weeks ago | 18 (10.7) | 14 (12.7) | 4 (6.9) | 13 (16) | 5 (5.7) |
| Last month | 34 (20.2) | 17 (15.5) | 17 (29.3) | 16 (19.8) | 18 (20.7) |
| More than a month ago | 106 (63.1) | 73 (66.4) | 33 (56.9) | 46 (56.8) | 60 (69) |
| Covid-19 outcome | |||||
| Hospitalized | 23 (13.7) | 14 (12.7) | 9 (15.5) | 8 (9.9) | 15 (17.2) |
| Dead | 7 (4.2) | 5 (4.5) | 2 (3.4) | 3 (3.7) | 4 (4.6) |
| Quarantined | 138 (82.1) | 91 (82.7) | 47 (81) | 70 (86.4) | 68 (78.2) |
| ICU | 19 (11.1) | 110 (31.3) | 58 (27.4) | 81 (31.3) | 87 (28.6) |
| No | 149 (88.7) | 242 (68.8) | 154 (72.6) | 178 (68.7) | 217 (71.4) |
a Values are presented as No. (%).
4.3. History of COVID-19 Among Patients
Out of 144 (25.4%) patients who were tested for COVID-19, 61 (42.2%) had positive test results. Among patients with a positive test, 21 (34.4%) required oxygen therapy, 20 (32.8%) were hospitalized, and 6 (9.8%) were admitted to the intensive care unit (ICU) (Table 3).
| Overall | Sex | Age | |||
|---|---|---|---|---|---|
| Male | Female | ≤ 65 | > 65 | ||
| Symptoms in the past month | |||||
| Fever | 45 (7.9) | 18 (5.1) | 27 (12.7) | 21 (8) | 24 (7.9) |
| Fatigue | 97 (17.1) | 53 (14.9) | 44 (20.8) | 48 (18.4) | 49 (16.1) |
| Pain | 110 (19.4) | 60 (16.9) | 50 (23.6) | 51 (19.5) | 59 (19.3) |
| Shortness of breath | 43 (7.6) | 18 (5.1) | 25 (11.8) | 21 (8) | 22 (7.2) |
| Diarrhea | 20 (3.5) | 11 (3.1) | 9 (4.2) | 10 (3.8) | 10 (3.3) |
| Nausea | 19 (3.3) | 5 (1.4) | 14 (6.6) | 11 (4.2) | 8 (2.6) |
| Headache | 66 (11.6) | 33 (9.3) | 33 (15.6) | 36 (13.8) | 30 (9.8) |
| Imbalance | 111 (19.5) | 69 (19.4) | 42 (19.8) | 46 (17.6) | 64 (21) |
| Taste disorder | 19 (3.3) | 10 (2.8) | 9 (4.2) | 11 (4.2) | 8 (2.6) |
| Smell disorder | 27 (4.8) | 15 (4.2) | 12 (5.7) | 18 (6.9) | 9 (3) |
| Result of COVID-19 test | |||||
| Positive | 61 (42.4) | 37 (43) | 23 (40.4) | 37 (56.9) | 23 (29.9) |
| Negative | 83 (57.6) | 49 (57) | 34 (59.6) | 28 (43.1) | 54 (70.1) |
| Need to receive oxygen therapy | |||||
| Yes | 21 (34.4) | 11 (29.7) | 9 (39.1) | 10 (27) | 10 (43.5) |
| No | 40 (65.6) | 26 (70.3) | 14 (60.9) | 27 (73) | 13 (56.5) |
| Need to be hospitalized | |||||
| Yes | 20 (32.8) | 13 (35.1) | 6 (26.1) | 9 (24.3) | 10 (43.5) |
| No | 41 (67.2) | 24 (64.9) | 17 (73.9) | 28 (75.7) | 13 (56.5) |
| ICU | |||||
| Yes | 6 (9.8) | 3 (8.1) | 2 (8.7) | 4 (10.8) | 1 (4.3) |
| No | 55 (90.2) | 34 (91.9) | 21 (91.3) | 33 (89.2) | 22 (95.7) |
a Values are presented as No. (%).
4.4. Post COVID-19 Complications
Table 4 displays the post-COVID-19 complications. Accordingly, exacerbation of movement problems (21.3%), weight loss (19.7%), and urinary problems (14.8%) were the most common complications. However, 69% of patients did not report any complications.
| Post COVID Complications | No. (%) |
|---|---|
| Loss of consciousness | 5 (8.2) |
| Sexual dysfunction | 4 (6.6) |
| Exacerbation of imbalance | 8 (13.1) |
| Exacerbation of movement problems | 13 (21.3) |
| Thrombosis | 2 (3.3) |
| Hair loss | 5 (8.2) |
| Weight loss | 12 (19.7) |
| Weight gain | 1 (1.6) |
| Urinary problems | 9 (14.8) |
| Skin problems | 5 (8.2) |
| Gastrointestinal problems | 6 (9.8) |
| Other | 2 (3.3) |
| No problem | 42 (68.9) |
4.5. The Relation Between COVID-19 and Patient Characteristics
In the univariate logistic regression model (Table 5), blood group, age at onset, Levodopa, history of HTN, and history of Ibuprofen or Gelofen use have shown a significant association with COVID-19. Patients with blood group B had 3.5 times higher odds of COVID-19 compared to patients with blood group O. Every one-unit increase in the age at onset decreased the odds of being COVID-19 positive by 2% (OR = 0.980; 95% CI: [0.961 - 0.999]). The chance of COVID-19 was 2.2 times greater (OR = 2.289; 95% CI: [1.014 - 5.166]) for patients taking Levodopa than for patients receiving other treatments. Patients with a history of HTN had a lower risk of COVID-19 (OR = 0.879; 95% CI: [0.779 - 0.994]) than those without. Patients who had a history of Ibuprofen or Gelofen use were 2 times more likely to have COVID-19 compared with those who did not use this drug (OR = 2.022; 95% CI: [1.155 - 3.537]).
In the final multivariate logistic regression model (Table 5), blood group (OR = 3.417; 95% CI: [1.455 - 8.025]), age at onset (OR = 0.976; 95% CI: [0.953 - 0.999]), and Levodopa (OR = 3.672; 95% CI: [1.095 - 12.31]) were associated with COVID-19.
| Characteristics | Univariate | Multivariate | ||
|---|---|---|---|---|
| OR (95% CI) | P-Value | OR (95% CI) | P-Value | |
| Age | 0.981 (0.958 - 1.005) | 0.120 | ||
| Sex (Ref = Female) | 0.956 (0.551 - 1.658) | 0.873 | ||
| Marital status (Ref = Married) | 0.148 (0.020 - 1.093) | 0.061 | ||
| BMI | 1.048 (0.988 - 1.113) | 0.120 | ||
| Smoking (Ref = No) | 0.567 (0.170 - 1.886) | 0.355 | ||
| Age at onset | 0.980 (0.961 - 0.999) | 0.041 | 0.976 (0.953 - 0.999) | 0.037 |
| Disease duration | 1.036 (0.989 - 1.084) | 0.133 | ||
| Levodopa (Ref = No) | 2.289 (1.014 - 5.166) | 0.046 | 3.672 (1.095 - 12.31) | 0.035 |
| Amantadine (Ref = No) | 1.190 (0.906 - 1.564) | 0.212 | ||
| Peramipexole (Ref = No) | 1.175 (0.980 - 1.409) | 0.082 | ||
| Biperiden (Ref = No) | 0.669 (0.405 - 1.103) | 0.115 | ||
| Selegiline (Ref = No) | 0.944 (0.625 - 1.427) | 0.786 | ||
| Trehexyphenidyl (Ref = No) | 0.955 (0.839 - 1.088) | 0.490 | ||
| Vitamin D (Ref = No) | 1.469 (0.833 - 2.591) | 0.184 | ||
| CVD (Ref = No) | 0.625 (0.298 - 1.307) | 0.212 | ||
| DM (Ref = No) | 0.912 (0.647 - 1.286) | 0.600 | ||
| Asthma (Ref = No) | 0.859 (0.433 - 1.702) | 0.663 | ||
| Lung (Ref = No) | 1.017 (0.798 - 1.297) | 0.889 | ||
| HTN (Ref = No) | 0.879 (0.779 - 0.994) | 0.039 | 0.884 (0.754 - 1.036) | 0.127 |
| Cancer (Ref = No) | 1.006 (0.710 - 1.427) | 0.971 | ||
| Stroke (Ref = No) | 1.093 (0.973 - 1.228) | 0.134 | ||
| Kidney (Ref = No) | 1.062 (0.965 - 1.169) | 0.219 | ||
| Liver (Ref = No) | 1.048 (0.884 - 1.243) | 0.587 | ||
| Aspirin (Ref = No) | 1.214 (0.699 - 2.109) | 0.491 | ||
| Ibuprofen or Gelofen (Ref = No) | 2.022 (1.155 - 3.537) | 0.014 | 1.422 (0.699 - 2.891) | 0.331 |
5. Discussion
COVID-19 has been associated with complications in patients with underlying diseases since the beginning of the pandemic, and numerous studies have been published focusing on the effects of COVID-19 on Parkinson's disease (PD) (9). Previous studies have suggested a poorer outcome in patients with PD after contracting COVID-19 compared to the general population (10). We conducted this study to evaluate the prevalence of COVID-19 in PD patients and explore possible associations between COVID-19 and clinical features of PD.
There are conflicting results regarding the prevalence of COVID-19 among PD patients compared to the general population. Del Prete et al. reported a higher prevalence of COVID-19 among PD patients in Tuscany, Italy, compared to the general population (11). However, other studies have reported lower or similar prevalence rates (12, 13). A recent study conducted on 647 Iranian patients showed a lower prevalence of COVID-19 compared to an age-matched control group. The prevalence of COVID-19 was 11.28% among patients with PD, while it was 15.39% in the age-matched control group (14). The prevalence of COVID-19 among PD patients was 10.7% in our study, which is consistent with these findings. Khoshnood et al. conducted a systematic review and meta-analysis, estimating a 5% prevalence of COVID-19 in PD patients. The hospitalization rate was 49%, with a mortality rate of 12%. However, the study's high heterogeneity emphasized the necessity for comprehensive studies with larger sample sizes (15). These inconsistent results could be attributed to different methodologies, patient ethnicities, lockdown situations, and variations in healthcare systems. PD patients might exercise extra caution due to their underlying disease and adhere strictly to social distancing and isolation protocols, potentially resulting in the lower prevalence of COVID-19 reported in some studies (16). Factors such as limited access to diagnostic tests and healthcare in impoverished countries, combined with inadequate disease transmission control due to social and economic reasons, can introduce heterogeneity into the results. This diversity makes it challenging to draw overarching conclusions on this matter.
In our study, the most common comorbidities were hypertension (HTN), cardiovascular disease (CVD), and diabetes mellitus (DM). Patients with HTN were at a lower risk of COVID-19 compared to those without HTN. Moreover, PD patients are often elderly, and older individuals tend to have poorer outcomes with COVID-19 (10). This result does not necessarily imply a positive correlation between HTN and COVID-19. Instead, it may be attributed to the higher prevalence of hypertension in the elderly. Given that negative outcomes of COVID-19 are less pronounced in this age group, fewer consequences were observed. However, in younger individuals, the coexistence of Parkinson's and hypertension could potentially exacerbate the infection's progression and spread, considering the adverse effects of hypertension and the poor prognosis associated with COVID-19 in such patients (17, 18).
While this study didn't reveal a notable association between DM and COVID-19 infection in multivariate analysis, the overall impact on infection and its spread in patients with both Parkinson's and DM was more severe than in those with Parkinson's alone. Furthermore, the likelihood of superinfection in Parkinson's patients with DM exceeded that in those without DM, contributing to the compounded negative consequences of COVID-19 in the diabetic group and showcasing a potential synergistic effect with Parkinson's and COVID-19 (18, 19).
In our study, among the 61 patients who were COVID-19 positive, 34.4% required oxygen therapy, 32.8% were hospitalized, and 9.8% were admitted to the ICU. Previous studies suggest that PD patients are often hospitalized due to the severity of COVID-19, but the rate of hospitalization in these patients is not higher compared to the normal population (20). Similarly, Salari et al. reported that the hospitalization rate in PD patients was even lower than in the normal population (14).
Regarding post-COVID-19 complications, the majority of the patients (69%) in our study did not experience any complications. However, 21.3% reported motor problems, 19.71% reported weight loss, and 14.8% complained about urinary problems. Some studies have stated that there is no significant association between COVID-19 and motor symptom deterioration (11). On the other hand, other studies have reported that more than half of the patients experienced worsening motor symptoms (13). Anxiety, isolation, prolonged inactivity, and sudden discontinuation of anti-Parkinson drugs are among the possible causes that trigger the motor symptoms of patients.
There was a significant association between blood types and the risk of COVID-19 in our study. Patients with blood type B were 3.5 times more likely to test positive for COVID-19 compared to those with blood type O. Previous studies show a slightly increased prevalence of COVID-19 in non-O blood types in the normal population (13). However, the association between blood types and the risk of COVID-19 was not evaluated previously.
Our study showed that the risk of COVID-19 in patients taking Levodopa was 2.2 times greater than in those taking other treatments, such as Amantadine (14). Amantadine is hypothetically proposed as a beneficial drug for COVID-19 due to its antiviral properties. However, there is not enough clinical data to support this hypothesis (21).
Neuropathologically, there is insufficient evidence supporting the entry of the SARS-CoV-2 virus into neurons and neuroglia. Despite the potential facilitation of virus entry by increasing angiotensin-converting enzyme 2 (ACE2) expression, no study has demonstrated elevated ACE2 levels in neurons during COVID-19 infection. This diminishes the likelihood of a direct connection between SARS-CoV-2 and the destruction of the Substantia nigra pathway to the corpus striatum (10). Some studies propose that the progression of Parkinson's may be influenced by the increased inflammation induced by the presence of SARS-CoV-2 (22, 23).
In summary, it is clear that, like many other chronic diseases, the clinical features of PD change during the course of COVID-19 infection. Although many studies have been published to evaluate the clinical characteristics of PD in different populations, there is still no consensus on the results and various reasons contributing to that. Among all the current studies, our study had the most similarity to the study by Salari et al. since they were both carried out in Iran and they shared a similar methodology (14). Additionally, our study proposed some new features, such as the association between blood type, Levodopa, and age at onset with COVID-19, that need to be further investigated. Given this study's limitation in examining the long-term complications of COVID-19 in patients, it is advisable to undertake cohort studies with extended follow-up periods to provide a more comprehensive understanding.
5.1. Study Limitations
This study encountered several limitations. Firstly, the memory status of Parkinson's patients posed a challenge, as the aging population might experience recall bias, impacting the accuracy of recorded events related to COVID-19 infection. Despite efforts to verify information through interviews with key individuals, the complete elimination of recall bias remained unattainable. Additionally, the study faced limitations due to incomplete checklist responses from elderly patients. The inability to manage confounding factors in diagnosing and treating COVID-19 in Parkinson's patients. Cost constraints and limited healthcare access might have led to the potential underrepresentation of cases. Moreover, the study's cross-sectional design hindered the exploration of long-term complications arising from COVID-19 infection in Parkinson's patients.