1. Background
Coronavirus disease 2019 (COVID-19) is caused by a novel coronavirus called severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). The virus can lead to various symptoms which differ in severity among those affected (1). The primary symptoms of the disease include fever, cough, and difficulty breathing (2). Other respiratory symptoms, such as sore throat, might also occur (3). Additionally, individuals with COVID-19 might experience tiredness, headaches, loss of smell or taste (known as anosmia or ageusia), and gastrointestinal symptoms, such as vomiting, nausea, and diarrhea (4, 5). It is necessary to note that some infected individuals might not show any symptoms; however, others with existing health conditions or older age might face more severe complications (6).
In severe cases of SARS-CoV-2 infection, individuals are at elevated risk of developing pneumonia, acute respiratory distress syndrome (ARDS), and organ failure (7). Although the primary target of the virus is the respiratory tract, some studies have indicated that the virus might enter the nervous system through various pathways (8, 9). Interestingly, there are reports indicating that SARS-CoV-2 exhibits neurotoxic and neuro-invasive characteristics, potentially allowing it to enter the central nervous system (CNS). This observation might imply possible connections with neuroimmune disorders, such as multiple sclerosis (MS) (10-13).
Multiple sclerosis is an autoimmune condition characterized by inflammation and damage to nerve fibers of the nervous system (14). The clinical spectrum of MS encompasses a wide range of symptoms, including fatigue, gait difficulties, muscular weakness, paresthesia, impaired coordination, and cognitive impairments (15). Different clinical courses have been identified within the context of sclerosis, including relapsing-remitting MS, secondary progressive MS, and primary progressive MS (16).
The precise molecular mechanisms through which SARS-CoV-2 affects MS are under investigation (17). There are two possible routes through which SARS-CoV-2 can access the CNS. The first route involves the olfactory route, where SARS-CoV-2 directly infects the olfactory epithelium (18). The second is the hematogenous route. Severe acute respiratory syndrome coronavirus 2 might be inhaled into the lungs, reach the alveoli, and subsequently enter the bloodstream. Neuroinvasion through a hematogenous route can occur following a breach of the blood-brain barrier (BBB). Once within the brain parenchyma, both neurons and glial cells, including astrocytes, have been demonstrated to be susceptible to direct infection (19, 20). In the CNS, the presence of SARS-CoV-2 could disrupt processes, such as demyelination/remyelination, neurodegeneration, neuroinflammation, and synaptic loss in neurons (21). This disruption might contribute to the progression of MS. Several potential mechanisms exist that could contribute to the development of MS as a result of SARS-CoV-2 infection.
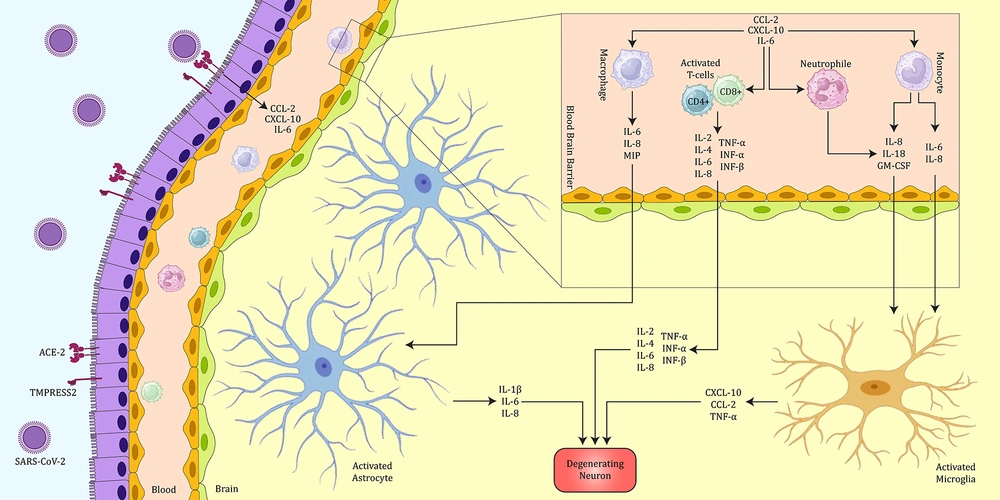
The first theory is cytokine storm and neuroinflammation. A cytokine storm is defined as a crucial immune reaction that triggers the excessive activation and multiplication of immune cells, accompanied by the activation of glial cells in the CNS, leading to neuroinflammation and demyelination (21-23). The entry of SARS-CoV-2 might trigger a cytokine storm and heightened demyelination by activating immune cells, such as macrophages, T-cells, and glial cells. This activation results in an increased expression of various cytokines, interleukins, and chemokines, ultimately leading to demyelination (Figure 1) (21). Additionally, the occurrence of a cytokine storm in individuals infected with SARS-CoV-2 might trigger the differentiation of T helper 17 (Th17) cells specific to CNS antigens. The heightened expression of interleukin-6 (IL-6), interleukin-17 (IL-17), and tumor necrosis factor-alpha (TNF-α) is noteworthy, given their significant association with MS (24).
The probable mechanism of COVID-19 in CNS results in inflammation and damage to the brain. In this figure, the possible mechanism of damage to neurons by SARS-CoV-2 is shown. Following the entry of the virus into the body and the activation of the immune system, CD4+ and CD8+ cells secrete various cytokines, especially TNF-α and IL-6, which can cause inflammation. In addition, the activation of innate immune cells, such as macrophages and neutrophils, can cause the secretion of IL-6 and IL-1β and subsequently intervene in the exacerbation of inflammation and neuronal damage. In addition, activated astrocytes can also be effective in worsening the condition of neuronal damage by secreting IL-1β and IL-6.
The second hypothesis involves hypoxia-induced mitochondrial dysfunction and a diminished ability to phagocytose myelin sheath debris. In this scenario, SARS-CoV-2 could potentially reduce the phagocytic capacity of microglia cells and macrophages for myelin sheath debris. The buildup of myelin sheath debris could impede the access of remyelinating cells, such as Schwann cells, thereby contributing to the development of MS (21, 24). Furthermore, it is crucial to emphasize that various reports have indicated the virus's potential for direct interaction with the CNS. This is attributed to the expression of the angiotensin-converting enzyme-2 (ACE-2) receptor in multiple tissues throughout the human body, encompassing the CNS. The receptor is predominantly identified in glial cells and neurons. Therefore, in the event that the virus accesses the CNS, neurons and glial cells become potential targets (25).
2. Objectives
The main objective of this study was to investigate how COVID-19 presents its clinical manifestations in individuals with MS and examine the differences in symptoms between those who have been infected by the virus. Moreover, this study aimed to understand how age and gender can impact the likelihood of contracting COVID-19 and the mortality rates associated with it among individuals diagnosed with MS.
3. Methods
3.1. Data Collection
The data collection took place at six hospitals supervised by the Iranian Network for Research in Viral Diseases (INRVD), which included Amiral Momenin, Imam Khomeini, Razi, Shariati, Sina, and Hajar, between March 2020 and July 2021. A consent letter was obtained from each patient who participated in the study, and it was approved by the Ethics Committees of Tehran University of Medical Sciences, Tehran, Iran (IR.TUMS.VCR.REC. 1399.599).
This study investigated a group of 63 individuals diagnosed with MS who had sought medical attention at the above-mentioned hospitals due to respiratory symptoms. Among them, 34 individuals (54%) were identified as female; however, 29 individuals (46%) were categorized as male. The individuals were categorized into two sets: those who were positive for COVID-19 and positive for MS (consisting of 30 individuals), and those who were negative for COVID-19 but positive for MS (comprising 33 individuals), who had been referred to the hospital for respiratory symptoms. Various aspects, including the variation in the intensity of clinical manifestations and the impact of age and gender on patients, were examined. Moreover, samples were collected in each hospital for further molecular assay. In the current study, all cases underwent comprehensive evaluation for active blood-borne viruses using enzyme-linked immunosorbent assay (ELISA). Rigorous testing was employed, and any suspected cases were systematically ruled out, ensuring a thorough and accurate assessment of the participants' viral status.
2.2. Molecular Assay
As per the guidelines set by the Iranian Center for Disease Control and Prevention (CDC), confirmation of SARS‐CoV‐2 infection involved conducting real‐time reverse transcription‐polymerase chain reaction (RT‐PCR) tests on throat samples using specialized flocked swabs. These swabs were promptly collected upon admission. In adherence to collaborative clinical virology standards, two sets of primers targeting two specific genes (E and RdRP genes) were employed (26). The Iranian Pasture Institute supplied these dual-target detection kits alongside a standardized protocol for implementation in laboratories nationwide. If the respiratory samples from patients tested positive for either or both genes, the specimens were classified as positive, confirming the case through laboratory analysis.
2.3. Statistical Analysis
To describe the data, number, percentage and median were calculated. The Mann-Whitney t-test was performed to test equality of age in the positive COVID-19 group and the negative COVID-19 group. Fisher’s exact test was used to test the dependence or independence of binary variables, such as baseline characteristics and COVID-19 symptoms, on study groups. Statistical package SPSS-27 was applied for analysis. Odds ratios were calculated and tested using the R 4.2.3 package.
3. Results
A total of 63 individuals diagnosed with MS were included in the study. In this study, 29 (46%) and 34 (54%) subjects were male and female, respectively. Moreover, 30 subjects (47.6%) were positive for COVID-19, and 33 patients were negative for COVID-19 but positive for MS. Most of the study subjects (93.7%) were from Tehran province. The proportion of gender groups did not differ significantly in positive/negative COVID-19 groups (chi-square value = 0.009, P = 0.923). The patients’ ages were recorded in some categories. The most frequent age category was 40-45 years old, and 22.2% of subjects were in this category. The frequencies of other categories are reported in Table 1. Patients with positive COVID-19 were significantly younger than patients with negative COVID-19 (Mann Whitney U = 212.0, P < 0.001), with 59.6 (standard deviation [SD] = 9.77) years in the dyslipidemia group and 52.9 (SD=9.73) years in the control group (Table 1).
| Age Range | SARS-CoV-2 | Total | |
|---|---|---|---|
| Negative | Positive | ||
| 30 – 35 | 1 (3.0) a | 3 (10.0) | 4 (6.3) |
| 35 – 40 | 1 (3.0) | 3 (10.0) | 4 (6.3) |
| 40 – 45 | 3 (9.1) | 11 (36.7) | 14 (22.2) |
| 45 – 50 | 4 (12.1) | 7 (23.3) | 11 (17.5) |
| 50 – 55 | 7 (21.2) | 1 (3.3) | 8 (12.7) |
| 55 – 60 | 8 (24.2) | 4 (13.3) | 12 (19.0) |
| 60 – 65 | 4 (12.1) | 1 (3.3) | 5 (7.9) |
| 65 – 70 | 5 (15.2) | 0 (0.0) | 5 (7.9) |
Abbreviation: SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
a Values are expressed as No. (%).
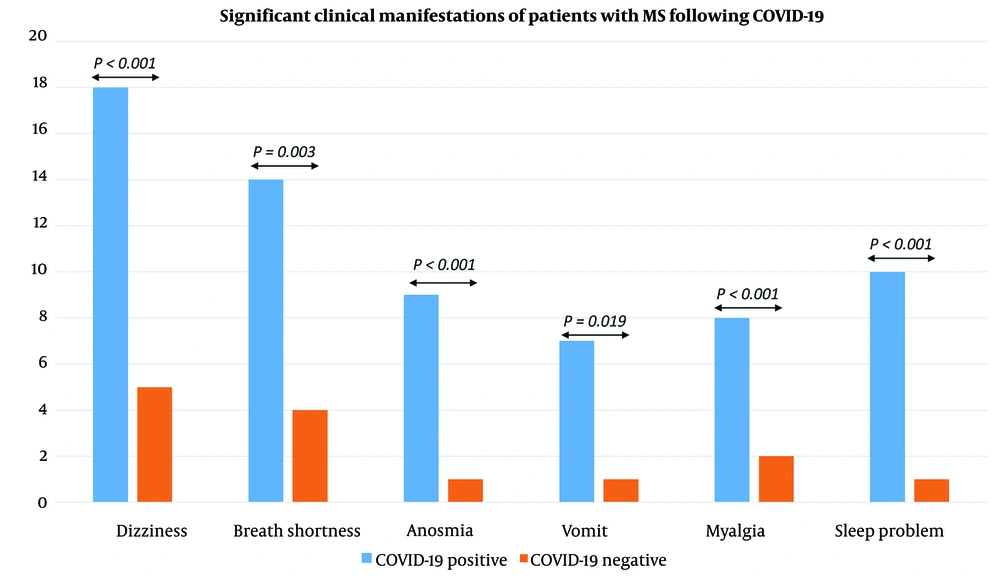
Finally, the frequency of each COVID-19 symptom was calculated separately for patients with positive COVID-19 and patients with negative COVID-19. The results are presented in Table 2. Odds ratios were also calculated for those symptoms, which were significantly dependent on the group.
| Clinical Symptoms | COVID-19 (% of Positive Subjects) | P-Value b | |
|---|---|---|---|
| Positive (n = 30) | Negative (n = 33) | ||
| Dizziness | 18 (60) | 5 (15.2) | < 0.001 c |
| Breath shortness | 14 (46.7) | 4 (12.1) | 0.003 c |
| Cough | 20 (66.66) | 22 (66.66) | 0.305 |
| Anosmia | 9 (30) | 1 (3) | < 0.001 c |
| Fatigue | 22 (73.33) | 7 (21.21) | 0.305 |
| Sore throat | 9 (30) | 4 (12.1) | 0.075 |
| Rhinorrhea | 7 (23.3) | 3 (9.1) | 0.115 |
| Vomit | 7 (23.3) | 1 (3) | 0.019 c |
| Fever | 5 (16.7) | 4 (12.1) | 0.437 |
| Delusion | 4 (13.3) | 2 (6.1) | 0.291 |
| Headache | 3 (10) | 1 (3) | 0.271 |
| Diarrhea | 2 (6.7) | 1 (3) | 0.464 |
| Sleeping problems | 10 (33.33) | 1 (3) | < 0.001 c |
| Myalgia | 8 (26.66) | 2 (6.1) | < 0.001 c |
a Values are expressed as No. (%).
b Fisher’s exact test was performed.
c Significant at 5% type I error.
According to Table 2, dizziness was significantly dependent on the group (P < 0.001). The odds of dizziness in positive COVID-19 patients were 8.4 times higher than in negative COVID-19 patients (Wald’s test statistics = 3.48, P < 0.001). The 95% confidence interval of the odds ratio for dizziness was 2.53-27.88. Breath shortness differed significantly between positive COVID-19 patients and negative COVID-19 patients (P = 0.003). The odds of having this symptom in positive COVID-19 patients were 6.4 times higher than negative COVID-19 group (Wald’s test statistics = 2.86, P = 0.007). The 95% confidence interval of the odds ratio for breath shortness was 1.79 - 22.54. Having a vomiting symptom depended significantly on the group (P = 0.019). The odds of vomiting in patients with positive COVID-19 were 9.7 times higher than negative COVID-19 group (Wald’s test statistics = 2.06, P = 0.047). The 95% confidence interval of the odds ratio for vomiting was 1.12-84.68, which is very wide. The risk of death due to COVID-19 was significantly higher among the positive COVID-19 group than the negative COVID-19 group. Moreover, 7 patients (23.3%) in the positive COVID-19 group died due to COVID-19; nevertheless, no COVID-19 death was observed in the negative COVID-19 group. Death consequence was statistically dependent on the group (P = 0.004). The odds of death due to COVID-19 in the positive COVID-19 group were 21.4 times higher than in the negative COVID-19 group (Wald’s test statistic = 2.062, P = 0.047). The main findings of the clinical symptoms, which show a significant P-value, are shown in Figure 2.
5. Discussion
The various respiratory manifestations of COVID-19 are widely recognized, spanning from mild symptoms to severe hypoxia accompanied by ARDS (27). However, some studies demonstrated that this virus can also impact other organs, including the nervous system (28). Study findings suggest that immunosuppression in certain instances might be correlated to worsened symptoms of COVID-19 and changing laboratory marker levels (29). Multiple sclerosis, which is characterized by persistent inflammation in the CNS, is particularly important in this context, given the immediate and delayed effects of SARS-CoV-2 on the nervous system. Consequently, unraveling the complex relationship between SARS-CoV-2 infection and MS has important implications for the realm of scientific understanding (30, 31). Comparing clinical symptoms can provide insights into its association with this high-risk group.
This study investigated the clinical symptoms of COVID-19 in patients with MS, compared to the non-infected group. The most frequent clinical manifestations among MS patients with COVID-19 infection were dizziness, anosmia, breath shortness, fatigue, vomiting, sleeping problems, and other symptoms. These symptoms are more frequent among MS patients with COVID-19 than the non-infected group (Table 2). The aforementioned results might suggest the potential link between COVID-19 and the exacerbation of MS symptoms. Additionally, the current analysis indicated that the impact of gender on COVID-19 infection risk among MS patients is not statistically significant, and younger might be more susceptible to COVID-19.
There are some reports which indicate the presence of respiratory symptoms, including dyspnea/shortness of breath and cough among MS patients with COVID-19 infection. Chaudhry et al. performed an investigation in 2020 to recognize the clinical features of MS related to worse COVID-19 outcomes. They carried out a prospective cohort study involving multiple centers to investigate the results of 40 individuals with confirmed COVID-19 who had been diagnosed with MS. In line with the current observation, the predominant respiratory symptoms among individuals with MS and COVID-19 infection in the aforementioned study included shortness of breath and cough, exhibiting rates of 50% and 65%, respectively (32).
In another investigation conducted in 2020 involving a total of 347 individuals diagnosed with both MS and COVID-19, the findings indicated that the prevalent respiratory symptoms associated with COVID-19 were dyspnea (46.7%) and cough (76.7%) (33). Furthermore, a survey carried out in 2022 in Egypt revealed that among individuals with MS, the prevalent COVID-19 symptoms following general manifestations, such as fever, headache, malaise, and anorexia, were chest symptoms, such as dyspnea and cough (34). According to a systematic review conducted in 2021 to examine existing literature on COVID-19 in individuals diagnosed with MS, it was demonstrated that among individuals with MS and COVID-19, 63% experienced cough; however, 39.5% reported shortness of breath or dyspnea, which is also spotted in the current analysis (35).
In addition to these complications, diverse neurologic symptoms, including dizziness, anosmia, depression, anxiety, and headache, have been reported in individuals diagnosed with both MS and COVID-19. In the present analysis, the most neurologic symptoms are dizziness (60%), sleeping problems (33.33%), and headache (10%). Similar to the current report, it is demonstrated that patients with MS recognized with COVID-19 exhibited symptoms, including dizziness (15.6%), headache (51.9%), and anosmia (43.2%) (33). Additionally, according to Parrotta et al.’s investigation, among 76 patients, 21.1% of them had neurologic symptoms following COVID-19 (36). Salter et al. performed research in 2021 to investigate the results and factors that contribute to the severity of COVID-19 in a sizable and varied group of North American individuals with MS (n = 1 626). The aforementioned report indicated that 144 patients (8.9%) experienced neurological symptoms (37). Furthermore, in another examination, it was demonstrated that among 39 MS patients with COVID-19, the distribution of psychiatric manifestations, such as depression and anxiety, is 10.25% (34). Correspondingly, several studies have reported neurologic symptoms in individuals diagnosed with both MS and COVID-19 (38-40).
In the context of other autoimmune disorders, a study of systemic lupus erythematosus (SLE) patients revealed that this group might be at an increased risk of developing severe disease from COVID-19. Some studies have suggested that this elevated risk might be particularly significant in older individuals with SLE, who are more likely to experience severe symptoms and require hospitalization following COVID-19 (41, 42). In addition, reports suggest that clinical symptoms experienced by these individuals might be altered following infection with COVID-19. Specifically, fever, anosmia (loss of sense of smell), and cough are the most common clinical manifestations of SARS-CoV-2 in patients with lupus (43, 44).
This study investigated whether contracting COVID-19 heightens the likelihood of mortality for individuals afflicted with MS. In general, it is observed that the likelihood of catching COVID-19 in individuals with autoimmune diseases is markedly greater than in those without such conditions. Moreover, it has been observed that autoimmune disease is linked to a 1.31-fold rise in the risk of mortality among patients affected by COVID-19 (45). The findings of the present study indicated that the risk of death due to COVID-19 among patients with MS is significantly greater than that of the non-infected cohort. The aforementioned results are consistent with previous reports detailing the elevated risk of COVID-19-related death among individuals with MS, which has been estimated to increase by 24% (46).
Although it should be noted that the results might vary due to the patient treatment status and the diverse stages of the disease, further studies with detailed information, including different strains of SARS-CoV-2 and larger statistical populations, are necessary to obtain more comprehensive insights.
5.1. Conclusions
The study examined the clinical manifestations of COVID-19 in individuals diagnosed with MS, aiming to discern differences in symptoms among those infected and those solely affected by MS. Among the obtained findings, the study noted that individuals with both MS and COVID-19 tended to be younger than those with MS alone. Additionally, certain symptoms, such as dizziness, breath shortness, and vomiting, were significantly more prevalent in individuals with both conditions. Furthermore, the risk of death due to COVID-19 was notably higher among those with both MS and the virus. The data unveiled a significant correlation between specific symptoms and the presence of COVID-19 in individuals diagnosed with MS. Notably, the study highlighted the heightened risk of severe outcomes and mortality correlated to COVID-19 in this particular population. Overall, the findings emphasize the significance of understanding the distinct clinical presentation of COVID-19 in individuals already diagnosed with MS, shedding light on potential correlations between the two conditions and the impact on disease severity and mortality.