1. Background
Epilepsy, a severe neurological condition, exhibits a lifetime prevalence of 7.60 per 1 000 persons and an incidence rate of 61.44 per 100 000 persons, with higher rates in low to middle-income countries (1). Despite the success of anti-epileptic drugs (AEDs) in controlling seizures for most patients, approximately 30 percent remain unresponsive (2, 3).
In a case-control study with a 3-year follow-up, drug-resistant epilepsy (DRE) patients were found to be prescribed an average of 5 AEDs, with a mortality rate three times higher than the control group (4). Studies indicate that with each failed AED regimen, the likelihood of achieving seizure freedom diminishes (5). Hence, DRE patients experiencing seizures that impede their social and occupational functioning are suitable candidates for surgical therapy (6), which has been shown to reduce mortality rates and improve the quality of life for epileptic patients (7, 8). The efficacy of surgery hinges on identifying a focal epileptogenic lesion through magnetic resonance imaging (MRI) scans (9). However, despite imaging advancements, 20 - 30% of temporal epilepsy and 20 - 40% of extratemporal epilepsy cases present with no discernible MRI lesions (10). In such instances, the localization of the epileptic zone relies on seizure semiology, scalp EEG, SPECT, and PET (10, 11).
The success rate of surgery for non-lesional patients is notably lower due to challenges in localizing the epileptogenic zone and potential overlap with major brain regions (12). Previous studies have reported varying seizure freedom rates for non-lesional epilepsy surgery, ranging from 41% to 65% for the temporal lobe, 37% for combined temporal and neocortical mesial sites, and 10% to 56% for extratemporal epilepsy, compared to 62 - 80% for lesional epilepsy (13). Although lesional epilepsy surgery yields better outcomes, a significant proportion of non-lesional epilepsy patients also achieve seizure freedom post-surgery. Hence, evaluating surgical outcomes for non-lesional epilepsy remains crucial (14).
Little research has been conducted on the outcomes of epilepsy surgery in the Middle East, leading to inadequate resources for establishing functioning epilepsy centers and a lack of sufficient knowledge among health professionals about epilepsy surgery as a therapeutic option. Additionally, the effects of non-lesional epilepsy surgery have not been explored in Iran.
2. Objectives
This study aimed to assess the surgical outcomes of epilepsy surgery in individuals with non-lesional DRE.
3. Methods
3.1. Study Design and Setting
This observational longitudinal study was conducted between August 2017 and February 2020 at an academic referral hospital, Imam Khomeini Hospital Complex, in Tehran, Iran.
3.2. Patients
Using the census sampling method, we screened 750 patient files. Inclusion criteria comprised intractable epileptic patients with non-lesional MRI findings who underwent surgical treatment for their epilepsy and consented to participate in the study. Intractable epilepsy diagnosis was based on ILAE guidelines (15), and non-lesional MRI was defined as normal or showing nonspecific white matter abnormalities and/or diffuse cerebral atrophy. Exclusion criteria included patients with lesional MRI, those who did not undergo epilepsy surgery, and individuals with incomplete medical records. Patients were monitored for two years post-surgery, with the initial follow-up visit scheduled within 14 days post-surgery.
3.3. Pre-operative Evaluation
Pre-surgical assessments included patient history, inpatient 24-hour continuous EEG monitoring, scalp EEG, physical examinations, neuropsychological and ophthalmological assessments, and MRI with epilepsy protocol, as per file data. MRI with epilepsy protocol is a specialized MRI designed for epileptic patients, providing detailed information about subtle mass lesions or cortical development malformations. It comprises specific sequences:
1. T1-weighted sagittal gradient-echo imaging with no intervening gap and 1.5 mm slice thickness.
2. Coronal, sagittal, and axial fluid-attenuated inversion recovery (FLAIR) with 0 - 1 inter-slice gap and 2 - 3 mm slice thickness.
3. Conventional thin-slice (3 mm), T2-weighted, axial and coronal sequences. Additional axial, coronal, and sagittal 3D FLAIR sequences can also be obtained (16).
3.4. EEG Recording
All cases underwent scalp EEG recording and 24-hour continuous EEG monitoring. Invasive electroencephalography recordings were obtained using a standard clinical system with a 256-Hz sampling rate (Nicolet, Natus Medical Inc., Pleasanton, CA, USA). Stereotactic EEG (SEEG) electrodes (diameter 0.86 mm, 8 or 10 contacts spaced 1 mm apart, 2 mm long) were implanted to cover the presumed epileptogenic region and investigate the relationship between the supposed epileptogenic region and the eloquent cortex. Extensive video-EEG monitoring with 31 scalp electrodes was conducted to record habitual seizures. The scalp EEG pattern was classified based on interictal epileptiform discharges (IEDs): Localized IEDs, lateralized but not localized IEDs, generalized or bilateral IEDs, and absence of IEDs.
3.5. Neuropsychological Evaluation
All cases underwent neuropsychological evaluations, including pre-operative neuropsychological assessments, which comprised the following tests:
1. Wechsler Intelligence Scale (WISC-1V) for general cognitive abilities.
2. Beck Depression Inventory for mood/social-affective processing.
3. Digit span memory test for attention and working memory.
4. Rey auditory verbal learning test (RAVLT) for verbal memory.
5. Brief visual memory test-revised for nonverbal/visual memory.
6. Wechsler constructional Praxis test score and clock drawing test for visuoperceptual processing.
7. Symbol digit modality test, delis–kaplan executive function system (DKEFS) trail making test for executive function.
8. DKEFS design fluency test for motor speed.
9. Persian naming test score and DKEFS verbal fluency test for language.
3.6. Surgical Procedure
Surgery decisions were made by a group of neurologists, neurosurgeons, and neuroradiologists based on clinical, neuroimaging, and 24-hour video EEG findings. Patients underwent four types of surgery: Temporal epilepsy surgery, extratemporal epilepsy surgery, Corpus callosotomy, and Vagus nerve stimulation (VNS).
3.7. Postoperative Evaluation
Postoperative evaluation included MRI, scalp EEG, neurologic examination, and clinical assessment by a neurologist and neurosurgeon using ILAE classification. Patients were regularly monitored for two years post-surgery, with the initial follow-up visit scheduled within 14 days of surgery. Antiepileptic drugs (AEDs) were adjusted based on follow-up visits and evaluations. The protocol for tapering AEDs, the number of follow-up sessions, and post-surgery MRI or EEGs were individualized based on clinical assessment.
3.8. Data Collection
We enrolled 80 patients according to the inclusion criteria. Age, gender, birth history, seizure risk factors, seizure onset age, seizure frequency, epilepsy duration, seizure type, family history of seizures, history of central nervous system infection, history of brain injury, history of developmental delay, prenatal complications, history of febrile convulsions, presence of pre-operative aura, and presence of pre-operative generalized tonic-clonic seizures were collected using a self-reported questionnaire and patients' files. Final long-term outcomes were graded using the ILAE classification. For statistical evaluation, we divided patients into four groups: ILAE classes I as seizure-free (SF), ILAE classes II, III, and IV as not seizure-free (NSF). We also collected complications related to surgery.
3.9. Statistical Analysis
All statistical analyses were conducted using IBM SPSS Statistics® version 26 (IBM Corp, Armonk, NY, USA). Mean and standard deviation were used for reporting quantitative variables, and frequency percentage was used to report qualitative variables. We used the chi-square, Fisher exact, and Kruskal-Wallis tests to determine the significant relationship between qualitative variables and the Mann-Whitney U test to compare quantitative variables. To adjust for possible confounding factors of surgery outcome, we used multiple binary logistic regression. Furthermore, only variables with a P-value less than 0.1 were entered into the regression. We handled missing data with pairwise deletion. P-values < 0.05 were considered statistically significant.
4. Results
4.1. Patient Characteristics
Of the 750 epilepsy patients, 88 met our inclusion criteria. Eight patients were lost to follow-up. Lastly, we included 80 patients, 38 (47.5%) male, and 42 (52.5%) female. Among the patients, 51 underwent temporal surgery, 12 underwent extratemporal surgery, 15 underwent corpus callosotomy surgery, and 2 underwent VNS. The mean ages for these groups were 33.22 ± 11.2, 27.83 ± 9.2, 17.73 ± 8.9, and 13 years, respectively. Table 1 shows the demographic and clinical characteristics of study participants.
| Variables | Surgery Type | |||
|---|---|---|---|---|
| Temporal (n = 51) | Extra Temporal (n = 12) | Corpus Callosotomy (n = 15) | VNS (n = 2) | |
| Age, y | 33.22 ± 11.2 | 27.83 ± 9.2 | 17.73 ± 8.9 | 13 |
| Gender, male/female | 25/26 | 5/7 | 8/7 | 0/2 |
| Epilepsy duration, mo | 17.3 ± 10.8 | 18.4 ± 8.2 | 15.7 ± 7.6 | 11 |
| Age at surgery, y | 32.41 ± 11.6 | 27.33 ± 8.8 | 17.07 ± 8.9 | 10.5 ± 3.5 |
| Seizure onset age, y | 15.44 ± 7.5 | 9.67±4.1 | 2.69 ± 3.09 | 1.5 ± 0.7 |
| Seizure type | ||||
| Autonomic | 17 (33.3) | 0 | 0 | 0 |
| Clonic | 1 (2) | 1 (8.3) | 1 (6.7) | 1 (50) |
| CPS | 8 (15.7) | 0 | 0 | 0 |
| CPS + GTC | 3 (5.9) | 1 (8.3) | 0 | 0 |
| GTC | 21 (41.2) | 5 (41.7) | 14 (93.3) | 0 |
| Hypermotor | 1 (2) | 5 (41.7) | 0 | 0 |
| Family history of seizure | 3 (5.9) | 2 (20) | 2 (13.3) | 0 |
| Birth history (NVD/CS) | 44/7 | 10/2 | 8/7 | 0/2 |
| Seizure risk factor | 9 (17.6) | 0 | 9 (60) | 1 (50) |
| History of CNS infection | 3 (5.9) | 0 | 1 (6.7) | 0 |
| History of brain injury | 6 (11.8) | 2 (16.7) | 9 (60) | 0 |
| History of developmental delay | 8 (15.7) | 0 | 1 (6.7) | 1 (50) |
| Prenatal complications | 3 (5.9) | 0 | 4 (26.7) | 1 (50) |
| Febrile convulsion | 10 (19.6) | 3 (25) | 6 (40) | 1 (50) |
| Involved hemisphere | ||||
| Left | 10 (19.6) | 5 (41.7) | 0 | 0 |
| Right | 39 (76.5) | 7 (58.3) | 0 | 0 |
| Both | 2 (3.9) | 0 | 15 (100) | 2(100) |
| Pre-operative aura | 25 (49) | 3 (25) | 0 | 0 |
| Pre-operative GTC | 44 (86.3) | 11 (91.7) | 15 (100) | 1 (50) |
| Seizure frequency | ||||
| Daily | 14 (27.5) | 9 (75) | 14 (93.3) | 1 (50) |
| Weekly | 35 (58.8) | 3 (25) | 1 (6.7) | 1 (50) |
| Monthly | 2 (13.7) | 0 | 0 | 0 |
| AED treatment | ||||
| Monotherapy | 4 (7.8) | 0 | 0 | 0 |
| Polytherapy | 47 (92.2) | 12 (100) | 15 (100) | 2 (100) |
| Pathological finding | ||||
| Mesial temporal sclerosis | 26 (51) | 0 | 0 | 0 |
| Gliosis | 1 (2) | 0 | 12 (80) | 1 (50) |
| Focal cortical dysplasia | 0 | 9 (75) | 0 | 0 |
| Others | 26 (51) | 3 (25) | 3 (20) | 1 (50) |
| Number of AEDs | 2.8 ± 1.08 | 3.5 ± 1.08 | 3.6 ± 1.2 | 4.5 ± 0.7 |
| Abnormal post-operative EEG | 17 (39.3) | 2 (16.7) | 15 (100) | 2 (100) |
| Follow-up duration, month | 16.4 ± 13.3 | 13.5 ± 8.9 | 13.9 ± 10.7 | 15.8 ± 10.3 |
Abbreviations: VNS, Vagus nerve stimulation; CPS, complex partial seizures; GTC, generalized tonic-clonic seizures; NVD, normal vaginal delivery; CS, cesarean section; CNS, central nervous system; AED, anti-epileptic drugs; EEG, electroencephalogram.
a Values are expressed as No. (%) or mean ± SD.
The mean duration of epilepsy before surgery was 17.3 ± 10.8, 18.4 ± 8.2, 15.7 ± 7.6, and 11 months in the temporal, extratemporal, corpus callosotomy, and VNS groups, respectively. Generalized tonic-clonic (GTC) seizures were the most common type of seizure among all groups. Aura was present in 25 (49%) patients of the temporal group, with psychic and epigastric auras being the most frequent (27.5% and 13.7%, respectively). Out of 3 extratemporal cases with aura, one case had a visual aura, and 2 cases had a psychiatric aura. Moreover, none of the participants in the corpus callosotomy and VNS groups had experienced an aura. 92.2% of patients in the temporal group and all patients in the other groups were treated with more than one AED.
4.2. Scalp EEG
In the Temporal epilepsy group, 40 (78.5%) patients had localized IEDs, one (2%) patient had lateralized but not localized IEDs, 8 (15.8%) patients had generalized or bilateral IEDs, and 2 (3.9%) patients had no IEDs.
In the Extratemporal epilepsy group, 5 (41.7%) patients had localized IEDs, 3 (25%) patients had lateralized but not localized IEDs, one (8.3%) patient had generalized or bilateral IEDs, and 3 (25%) patients had no IEDs.
In the Corpus callosotomy and VNS groups, all patients had generalized or bilateral IEDs.
4.3. Surgical Outcome
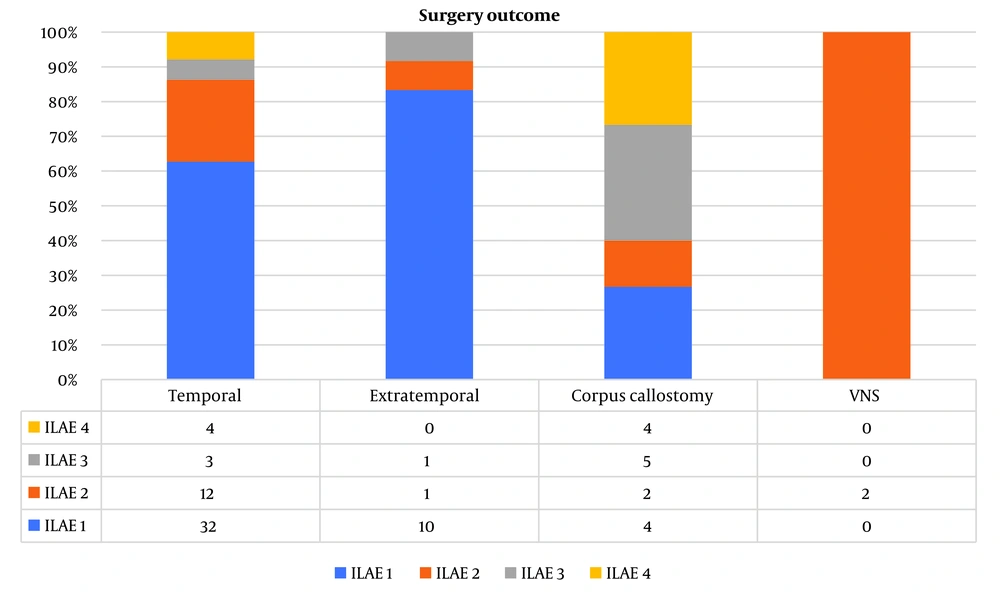
The mean follow-up duration in the temporal, extratemporal, corpus callosotomy, and VNS groups was 16.41 ± 13.3, 13.5 ± 8.9, 13.9 ± 10.7, and 13.2 months, respectively. Follow-up visits revealed that 32 patients (62.7%) in the temporal epilepsy group, 10 patients (83.33%) in the extratemporal group, and four patients (26.66%) in the corpus callosotomy group became seizure-free. However, none of the patients with the VNS procedure became seizure-free (Figure 1).
Post-operative EEG was normal in 28 patients (54.9%) in the temporal, 10 patients (83.3%) in
extratemporal, and one patient (50%) in the VNS groups. Nonetheless, none of the patients in the corpus callosotomy group had a normal post-operative EEG. Anti-epileptic drugs were discontinued in 20 patients (39.2%) in the temporal, 2 patients (16.7%) in extratemporal, and one (50%) patient in the VNS groups. Also, AEDs were reduced in 3 (5.9%) patients in the temporal and 4 (33.2%) patients in the extratemporal groups. However, in the corpus callosotomy cases, AEDs were continued in all patients.
4.4. Post-operative Complications
Surgical complications were observed in 4 (8%) patients with temporal epilepsy, including one patient with epidural hematoma (EDH), one with intracranial hemorrhage (ICH), one with an infection, and one patient with memory impairment. These individuals did not experience any significant long-term neurological deficits. Furthermore, all patients in the other groups did not report any surgical complications.
4.5. Potential Risk Factors
We compared pre-operative and post-operative baseline characteristics, seizure semiology, and lesion features between seizure-free (SF) and not seizure-free (NSF) patients in all groups.
There was no significant difference between SF and NSF patients in the temporal, extratemporal, and corpus callosotomy groups regarding epilepsy duration, age of seizure onset, age at surgery, seizure type, family history of seizure, birth history, seizure risk factors, history of CNS infection, history of brain injury, history of developmental delay, prenatal complications, febrile convulsion, involved hemisphere, presence of pre-operative aura, presence of pre-operative GTC, seizure frequency, number of AEDs, and pathological findings (Table 2).
| Outcome Variables | Surgery Type | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Temporal | Extratemporal | Corpus Callosotomy | |||||||
| SF (n = 32) | NSF (n = 19) | P-Value | SF (n = 10) | NSF (n = 2) | P-Value | SF (n = 4) | NSF (n = 11) | P-Value | |
| Age, y | 35.9 ± 10.4 | 29 ± 11.5 | 0.03 b | 28.2 ± 9.6 | 26 ± 9.8 | 0.74 | 19 ± 6.4 | 17.27 ± 9.8 | 0.90 |
| Male/female | 16/16 | 9/10 | 0.85 | 4/6 | 1/1 | 0.79 | 3/1 | 5/6 | 0.56 |
| Epilepsy duration | 12.6 ± 8.9 | 20.2 ± 11.02 | 0.006 b | 19.1 ± 9.1 | 15.5 ± 2.1 | 0.56 | 17.5 ± 7.0 | 15.05 ± 8.1 | 0.43 |
| Age at surgery | 27.5 ± 12.2 | 35.3 ± 10.4 | 0.99 | 27.9 ± 9.2 | 24.5 ± 9.1 | 0.61 | 18.75 ± 6.8 | 16.45 ± 9.7 | 0.34 |
| Seizure onset age | 16.2 ±9.9 | 15 ±5.8 | 0.99 | 9.8 ± 3.9 | 9 ± 7.07 | 0.79 | 1.25 ± 0.5 | 3.3 ± 3.5 | 0.60 |
| Seizure type | 0.87 | 0.92 | 0.53 | ||||||
| Autonomic | 9 (28.1) | 8 (41.2) | 0 | 0 | 0 | 0 | |||
| Clonic | 1 (3.1) | 0 | 1 (10) | 0 | 0 | 1 (9.1) | |||
| CPS | 6 (18.8) | 2 (10.5) | 0 | 0 | 0 | 0 | |||
| CPS + GTC | 2 (6.3) | 1 (5.3) | 1 (10) | 0 | 0 | 0 | |||
| GTC | 13 (40.6) | 8 (42.1) | 4 (40) | 1 (50) | 4 (100) | 10 (90.9) | |||
| Hypermotor | 1 (3.1) | 0 | 4 (40) | 1 (50) | 0 | 0 | |||
| Family history of seizure | 1 (3.1) | 2 (10.5) | 0.27 | 1 (10) | 1 (50) | 0.63 | 0 | 2 (18.2) | 0.36 |
| Birth history (NVD/CS) | 28/4 | 16/3 | 0.74 | 9/1 | 1/1 | 0.16 | 3/1 | 5/6 | 0.31 |
| Seizure risk factor | 6 (18.8) | 3 (15.8) | 0.41 | 0 | 0 | 0 | 2 (50) | 7 (63.6) | 0.63 |
| History of CNS infection | 1 (3.1) | 2 (10.5) | 0.27 | 0 | 0 | 0 | 0 | 1 (9.1) | 0.99 |
| History of brain injury | 5 (15.6) | 1 (5.3) | 0.26 | 1 (10) | 1 (50) | 0.16 | 3 (75) | 6 (54.5) | 0.47 |
| History of developmental delay | 3 (9.4) | 5 (26.3) | 0.108 | 0 | 0 | 0 | 4 (100) | 10 (90.9) | 0.53 |
| Prenatal complications | 1 (3.1) | 2 (10.5) | 0.27 | 0 | 0 | 0 | 2 (50) | 2 (18.2) | 0.21 |
| Febrile convulsion | 6 (18.8) | 4 (21.1) | 0.40 | 1 (10) | 2 (100) | 0.007 b | 3 (75) | 3 (27.3) | 0.09 b |
| Involved hemisphere | 0.43 | 0.19 | 0.99 | ||||||
| Left | 8 (25) | 2 (10.5) | 5 (50) | 0 | 0 | 0 | |||
| Right | 23 (71.9) | 16 (84.2) | 5 (50) | 2 (100) | 0 | 0 | |||
| Both | 1 (3.1) | 1 (5.3) | 0 | 0 | 4 (100) | 11 (100) | |||
| Pre-operative aura | 17 (53.1) | 8 (42.1) | 0.44 | 2 (20) | 1 (50) | 0.37 | 0 | 0 | - |
| Pre-operative GTC | 29 (90.6) | 15 (78.9) | 0.24 | 9 (90) | 2 (100) | 0.64 | 4 (100) | 11 (100) | - |
| Seizure frequency | 0.88 | 0.52 | 0.99 | ||||||
| Daily | 8 (25) | 6 (31.6) | 7 (70) | 2 (100) | 4 (100) | 10 (90.9) | |||
| Weekly | 24 (75) | 11 (42.1) | 3 (30) | 0 | 0 | 1 (9.1) | |||
| Monthly | 0 | 2 (10.5) | 0 | 0 | 0 | 0 | |||
| AED treatment | 0.77 | 0.72 | 0.99 | ||||||
| Monotherapy | 1 (3.1) | 3 (15.8) | 0 | 0 | 0 | 0 | |||
| Polytherapy | 31 (96.9) | 16 (84.2) | 10 (100) | 2 (100) | 4 (100) | 11 (100) | |||
| Pathological finding | 0.42 | 0.36 | 0.68 | ||||||
| Mesial temporal sclerosis | 14 (43.7) | 10 (52.6) | 0 | 0 | 0 | 0 | |||
| Gliosis | 0 | 1 (5.3) | 0 | 0 | 3 (75) | 9 (81.8) | |||
| Focal cortical dysplasia | 0 | 0 | 8 (80) | 1 (50) | 0 | 0 | |||
| Others | 18 (56.8) | 8 (42.1) | 2 (20) | 1 (50) | 1 (25) | 2 (18.2) | |||
| Number of AEDs | 3.03 ± 0.96 | 2.6 ± 1.2 | 0.16 | 3.3 ± 0.82 | 4.5 ± 2.12 | 0.18 | 3.75 ± 0.95 | 3.63 ± 1.4 | 0.64 |
| Abnormal post-operative EEG | 11 (34.4) | 8 (47.4) | 0.52 | 1 (10) | 1 (50) | 0.026 b | 4 (100) | 11 (100) | 0.76 |
Abbreviations: SF, seizure-free; NSF, not seizure-free; CPS, complex partial seizures; GTC, generalized tonic-clonic seizures; NVD: normal vaginal delivery; CS: cesarean section; CNS: central nervous system; AED, anti-epileptic drugs; EEG,electroencephalogram.
a Values are expressed as No. (%) or mean ± SD.
b Significant at P-value < 0.05.
In the temporal epilepsy group, the mean age of SF patients was significantly higher than NSF patients (35.9 ± 10.4 vs 29 ± 11.5, P-value = 0.03); this difference was not significant in extratemporal and corpus callosotomy groups (P-values = 0.74 and 0.90, respectively).
In the extratemporal surgery group, there was a significant difference in post-operative EEG between SF and NSF groups (P-value = 0.026), and also patients without a history of febrile convulsion were more likely to become seizure-free (P-value = 0.007); however, in extratemporal and corpus callosotomy groups, these differences were not significant (Table 2).
In the total population, univariate logistic regression analyses were conducted to examine the association between various predictor variables and seizure freedom. The results indicated that age (OR: 1.065, P-value = 0.005), corpus callosotomy (0.187, P-value = 0.011), involvement of the left hemisphere (OR: 3.946, P-value = 0.006), abnormal findings in postoperative EEG (OR: 0.25, P-value = 0.008), and a history of developmental delay (OR: 0.191, P-value = 0.002) demonstrated significant associations or odds with seizure freedom when analyzed individually (Table 3). However, upon entering these significant variables into a multiple logistic regression model, the associations were no longer significant (Table 4).
| Variables | Odds Ratio | P-Value |
|---|---|---|
| Gender | ||
| Male | 1 (reference) | - |
| Female | 0.833 | 0.690 |
| Age, y | 1.065 | 0.005 |
| Surgery type | 0.021 | |
| Temporal | 1 (reference) | - |
| Extratemporal | 1.545 | 0.551 |
| Corpus callosotomy | 0.187 | 0.011 |
| Family history of seizure | 0.165 | 0.115 |
| Seizure risk factor | 0.432 | 0.119 |
| Seizure onset age | 1.026 | 0.376 |
| Pre-operative GTC | 1.500 | 0.558 |
| Pre-operative aura | 2.199 | 0.118 |
| Involved hemisphere | 0.006 | |
| Right | 1 (reference) | - |
| Left | 3.946 | 0.094 |
| Both | 0.234 | 0.017 |
| Post-op abnormal EEG | 0.250 | 0.008 |
| Febrile convulsion | 0.611 | 0.346 |
| History of developmental delays | 0.191 | 0.002 |
| Variables | B | S.E. | Odds Ratio | P-Value |
|---|---|---|---|---|
| Age | 0.019 | 0.030 | 1.019 | 0.514 |
| Surgery type (reference: Temporal) | 0.730 | |||
| Extratemporal | 0.630 | 0.925 | 1.877 | 0.496 |
| Corpus callosotomy | -0.001 | 1.860 | 0.999 | 1.000 |
| Involved hemisphere (reference: Right) | 0.165 | |||
| Left | 2.164 | 1.144 | 8.704 | 0.059 |
| Both | 0.339 | 1.917 | 1.404 | 0.860 |
| Abnormal post-op EEG | -0.533 | 0.684 | 0.587 | 0.436 |
| History of developmental delay | -1.141 | 1.029 | 0.319 | 0.267 |
| Constant | -0.119 | 1.059 | 0.888 | 0.911 |
5. Discussion
In this study, we evaluated the outcomes of epilepsy surgery in non-lesional epileptic patients using the ILAE classification. During the pre-surgical evaluation of medically resistant epileptic patients, the absence of detectable lesions in an MRI complicates clinical decision-making and lowers surgical efficacy. Our study demonstrates that epilepsy surgery could be beneficial if we carefully select non-lesional patients. This finding is consistent with existing evidence reported by preliminary studies assessing non-lesional epilepsy surgery outcomes (17, 18).
In our study, none of the patients who underwent the VNS procedure became seizure-free. Due to the small number of patients in the VNS group (n = 2), we could not infer a conclusive result. However, several studies have reported low seizure freedom rates and only decreased seizure frequency using the VNS technique (19, 20). It is important to note that while seizure freedom is more probable with resection rather than neuromodulation methods such as VNS therapy, certain patients are not good candidates for resection, and neuromodulation should be considered afterward.
We found that the seizure freedom rate was 62.7% for temporal, 83.3% for extratemporal, and 26.7% for corpus callosotomy surgeries. This finding contrasts with other studies that have noted a relatively poor surgical outcome for extratemporal epilepsy surgery (29 - 56% success rate) compared to temporal epilepsy surgery (41 - 65% success rate) (21).
The effectiveness of temporal lobe epilepsy surgery is possibly attributable to the fact that temporal lobe epilepsy originates predominantly from the anteromedial temporal lobe, a network typically implicated in epileptogenesis, which is fully resected in a temporal lobectomy (22). The etiology of extratemporal epilepsy is congenital, such as cortical dysplasia. Thus, the development of seizures in these patients is less well-established. This makes developing a surgical procedure for non-lesional extratemporal epileptic patients challenging (18). Although we reported higher seizure freedom rates in extratemporal epilepsy, we believe this is because all patients with extratemporal epilepsy were followed longer.
This study demonstrated that individuals without a history of febrile convulsions and a normal post-operative EEG were more likely to achieve seizure-free status following extratemporal surgery. This suggests that abnormal post-operative EEG and a history of febrile convulsion can predict adverse outcomes in patients with extratemporal surgery. This finding contradicts a meta-analysis by Ansari, which found that none of the adult factors showed a significant association with outcome (23).
Previous research has demonstrated that even older individuals can benefit from epilepsy surgery, and age should not be a limiting factor for epilepsy surgery (24, 25). Our study not only discovered that age was not a limitation but also found that seizure-free patients were significantly older than non-seizure-free patients who underwent temporal epilepsy surgery.
Our study reported the surgical outcome of corpus callosotomy surgery. Corpus callosotomy is a palliative surgery that disconnects the two cerebral hemispheres and reduces the frequency and severity of seizures (26). Seizure freedom rates of corpus callosotomy surgery are reported to be 19% to 27.3%, which is consistent with our results (27, 28). Although we did not find any potential factors associated with becoming seizure-free in patients undergoing corpus callosotomy, previous studies have shown that a shorter epilepsy duration and infantile spasms predict a higher rate of complete seizure freedom in these patients (29).
One limitation of this study is not achieving a minimum 2-year follow-up for some patients. Another limitation is localizing the epileptogenic lesion with EEG and epileptic syndromes and not performing additional neuroimaging. The small sample size and inclusion of both adult and pediatric patients, which may increase heterogeneity, are other limitations of our study.
Despite these limitations, our study was the first to report non-lesional epilepsy surgery outcomes in Iran. We suggest that performing epilepsy surgery in developing countries with minimal resources is extremely valuable and can be attempted in approved centers. To advance research beyond observational studies and enhance study rigor, future investigations should prioritize controlled interventional designs, larger sample sizes, and longer follow-up durations. This could help generate patient selection criteria for non-lesional epilepsy surgery.
5.1. Conclusions
We showed that epilepsy surgery could be beneficial in non-lesional DRE patients, and extratemporal surgery had the best success rate followed by temporal surgery. We discovered that better surgical outcomes following temporal surgery are associated with older age and shorter epilepsy duration. In comparison, having no history of febrile convulsions and normal post-operative EEGs had been associated with better surgical outcomes after extratemporal surgery. Further studies are needed to generate patient selection criteria for non-lesional epilepsy surgery.