1. Background
Pituitary adenomas are the most common space-occupying lesions in the sellar region. From radiological aspect, adenomas greater than 10 mm are being considered macroadenomas and those less than 1 cm in diameter are categorized as microadenomas (1). In the past decade, endonasal transsphenoidal approach has tended to be the preferred surgical treatment of pituitary tumors. The main goal of transsphenoidal surgery in patients with macroadenomas is the decompression of optic apparatus. The extent of excision through this route depends on the size, invasiveness, and consistency of the tumor. In this regard, the main limitation of the microscopic transsphenoidal technique may be the hard consistency of the tumor (2, 3). Most adenomas are soft and easily curetted by suctioning. However, about 10% of macroadenomas are fibrous and poorly amenable to suction and curettage (3). In such cases, an adequate tumor resection may not be achievable via a transsphenoidal approach and a more extensive transsphenoidal approach or a second operation like transcranial surgery or radiation is often required (4-6). Therefore, a reliable preoperative predictor of the pituitary adenoma consistency may serve as a useful biomarker for neurosurgeons to plan proper surgical approach and technique, thus reducing surgical complications. Previous studies have evaluated conventional MRI features in the prediction of macroadenomas consistency and histologic features but the results had been inconsistent. Some authors have reported that macroadenomas with low T2-weighted signal intensity, as a measure of low water content (2, 3, 7), and more homogenous enhancement mostly happen to be dense, fibrous, and contain more collagen (8). On the contrary, others have found no correlation between tumor consistency and MRI findings (3, 9-11).
Diffusion weighted imaging (DWI) provides information on tissue water diffusion by visualization of Brownian motion of water molecules, which is affected by the size and integrity of structures (12). Proton motion can reflect microstructural properties of tissue, so DWI and computed apparent diffusion coefficient (ADC) may provide information about tumor consistency and resectability that cannot be reliably predicted by means of conventional MR techniques.
We hypothesized that DWI findings might be able to determine tumor consistency and identify unresectable tumors. Thus, the purpose of our study was to evaluate the role of preoperative DWI and ADC map in the prediction of macroadenoma structural integrity. Furthermore, the correlation of the tumor collagen content with the ADC value and the resectability of pituitary macroadenomas were also examined.
2. Methods
2.1. Study Population
All patients with pituitary macroadenomas who were referred to our hospital during 2 years from March 2011 to March 2013 were recruited in the study. Both secretory and non-secretory adenomas were included. Patients with a history of prior surgery, as well as cystic and hemorrhagic macroadenomas that might have resulted in lower ADC values were excluded. Consequently, a total of 45 patients (21 men, 24 women; age range, 23 - 60 years; mean age, 43 ± 10 (SD) years) met the inclusion criteria. The ethics committee at our institution approved the study protocol, and informed consent was obtained from all patients.
2.2. Imaging and Analysis
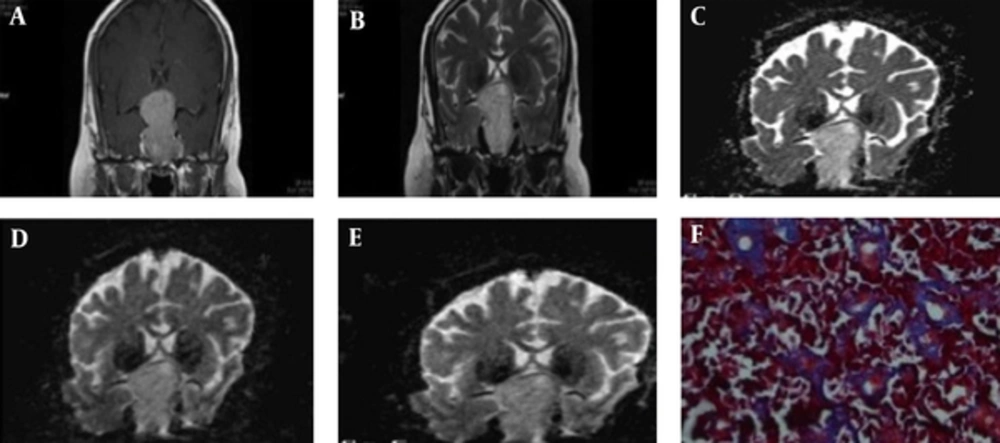
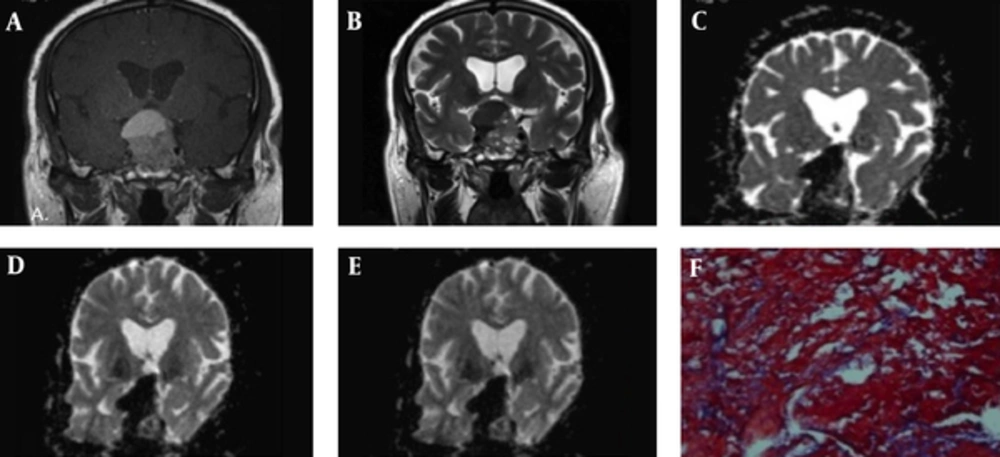
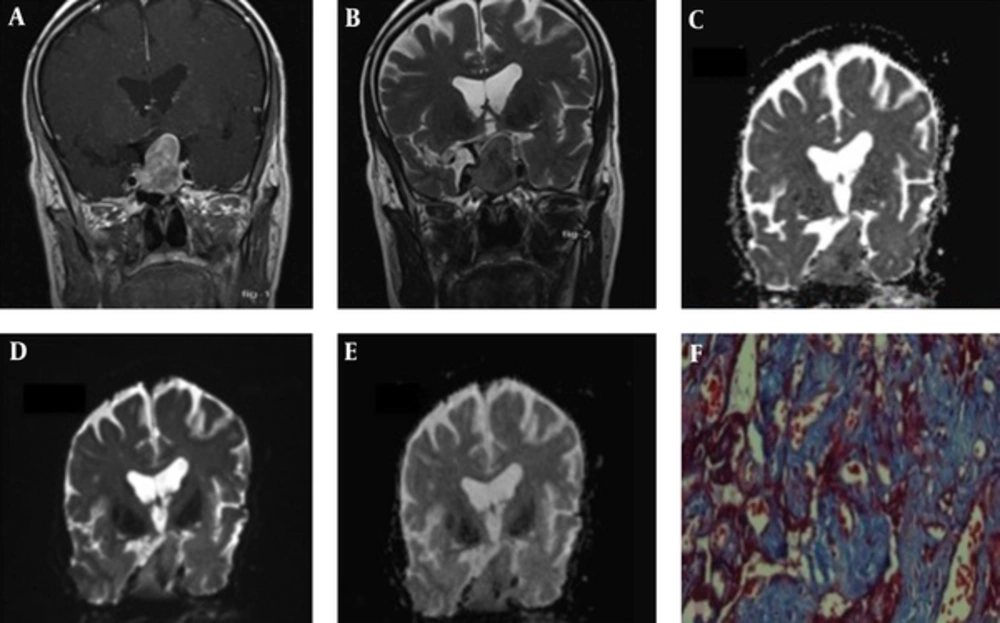
All patients underwent MR imaging with 3T clinical scanner (Magnetom Verio, Siemens Healthcare, Erlangen, Germany) equipped with a standard head coil. MRI protocol included axial and coronal T1-weighted images (TR 400 m/s, TE 17 m/s, FOV 20 × 20 mm, matrix 256 × 256, slice thickness of 3 mm with 1 mm gap) before and after IV administration of a single dose (0.1 mmol/kg) of gadoterate meglumine (Dotarem, GUERBET LLC) and axial and coronal T2-weighted images (TR 3000 m/s, TE 110 m/s, FOV 20 × 20 mm, matrix 256 × 256, slice thickness of 3 mm with 1 mm gap). Prior to contrast agent administration, DWI was acquired in the coronal and axial plane with a single-shot spin-echo echo-planar sequence (TR/TE: 5000/101-108, matrix size 256 × 256, section thickness 3 mm, intersection gap 1 mm, FOV 20 × 20 mm, and b values 0, 1000, 3000, and 5000 s/mm2). ADC maps were calculated from the raw DWI data automatically with the MRI unit console software. An expert neuroradiologist, who was blinded to surgical and histological outcome, measured the ADC value and signal intensity characteristics. ROIs were defined as the central and solid enhancing areas of the macroadenomas and small areas of cystic degeneration or hemorrhage were intentionally excluded. An equivalent ROI was selected within a normal white matter of temporal lobe. In conventional MR images, signal intensity of tumor on axial and coronal T1- and T2-Weighted images were measured and the relative SI of the macroadenomas was assessed with calculation of the ratio of SI in the tumor to the SI of normal white matter in the same patient. Axial and coronal mean ADC values were calculated using four different b-values (0, 1000, 3000, and 5000) in solid tumor and normal white matter for each patient as well as the ratio of tumor to normal white matter ADC was computed.
2.3. Clinical and Operative Data
Preoperatively, a comprehensive hormone assay was obtained in all patients. Surgical resection was performed via a microscopic transsphenoidal approach by the senior author (MZ), who was blinded to MRI and DWI findings. According to surgeon’s opinion during the surgery, tumors were categorized into 3 groups based on their consistency: soft (easily removable through suction), intermediate (removable by curettage but with difficulty through suction), or hard (non-removable).
2.4. Histological Analysis
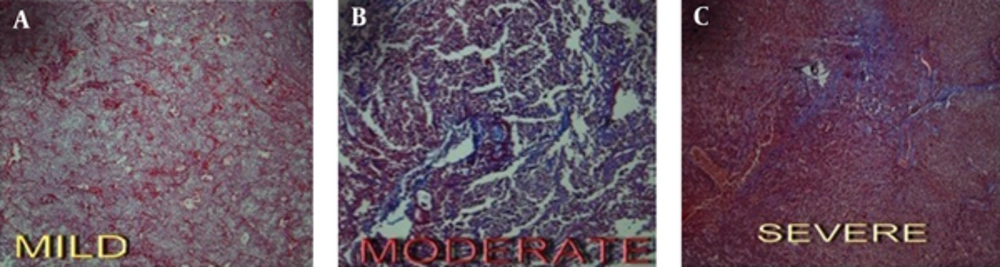
One pathologist examined the surgical specimens histologically in all patients while blinded to the clinical and MRI data. The tumor specimens were stained by hematoxylin and eosin (H and E) for routine examination and standard Masson’s trichrome to determine the collagen content of the samples. Collagen content was graded visually into three groups: mild, moderate, and severe (Figure 1).
Tumor specimen based on septal diameter was also classified as thin, moderate, and thick groups (Figure 2).
2.5. Statistical Analysis
Statistical analysis was performed using SPSS package (SPSS release 14.0 program, SPSS for windows; SPSS Inc, Chicago, IL). We assessed the correlation of the mean values of several quantitative MR parameters with the tumor consistency using Mann-Whitney U-test and analysis of variance. A P value less than 0.05 was considered statistically significant.
3. Results
All patients underwent microscopic transsphenoidal adenectomy. The mean time interval between onset of symptoms and surgery was 8.3 (± 3.1) months. We investigated the correlation between the relative SI and ADC and the consistency of the pituitary adenomas. Based on surgical findings, 12 (26.7%) tumors were classified as soft, 30 (66.6%) as intermediate, and 3 (6.7%) as hard macroadenomas.
Regarding pituitary hormonal status, GH-secreting adenomas (N = 4) showed an intermediate consistency in all cases. Also, in prolactinomas (N = 2), one case showed soft and the other one had an intermediate consistency. Among non-secreting adenomas (N = 38), there were 11 tumors with soft and 24 tumors with intermediate consistency, while we observed only 3 cases with hard consistency (Table 1). Of note, no significant difference was observed in ADC values and conventional MRI measures with the tumor consistency and excretory functions (Table 2).
| Variables | Tumor Consistency | P Value | ||
|---|---|---|---|---|
| Soft (N = 12) | Intermediate (N = 29) | Hard (N = 3) | ||
| Age, y | 42.50 ± 11.57 | 44.06 ± 9.52 | 42.66 ± 10.78 | 0.89 |
| Sex (female) | 7 (29) | 16 (66.7) | 1 (4) | 0.61 |
| Hormone-secreting status: | 0.80 | |||
| Non-functional | 11 (28) | 24 (61.5) | 3 (8) | |
| Acromegaly | 0 | 4 (100) | 0 | |
| Prolactinoma | 1 (50) | 1 (50) | 0 | |
| Collagen content | 0.05 | |||
| Mild | 5 (36) | 9 (64) | 0 | |
| Moderate | 6 (30) | 12 (60) | 2 (10) | |
| Severe | 0 | 7 (78) | 1 (22) | |
| Septal diameter | 0.46 | |||
| 1 | 5 (33.3) | 9 (60) | 0 | |
| 2 | 4 (23.5) | 12 (71) | 1 (6) | |
| 3 | 2 (18.2) | 7 (63.6) | 2 (18.2) | |
A Comparison of Clinical and Pathological Data Between Tumor Groups with Different Consistenciesa
| SI of Tumor to SI of Normal White Matter | Hormone-Secreting Status | P Value | ||
|---|---|---|---|---|
| Non-functional (N = 38) | GH-producing adenoma (N = 4) | Prolactinoma (N = 2) | ||
| Axial ADC, b value = 0 | 3.69 ± 10.17 | 1.14 ± 0.48 | 0.94 ± 0.74 | 0.73 |
| Axial ADC, b value = 1000 | 3.12 ± 8.88 | 0.96 ± 0.39 | 0.81 ± 0.36 | 0.80 |
| Axial ADC, b value = 3000 | 2.29 ± 6.04 | 1.01 ± 0.37 | 0.61 ± 0.17 | 0.43 |
| Axial ADC, b value = 5000 | 1.99 ± 3.59 | 1.14 ± 0.52 | 0.57 ± 0.03 | 0.38 |
| Coronal ADC, b value = 0 | 4.31 ± 20.88 | 1.20 ± 0.75 | 0.48 ± 0.16 | 0.44 |
| Coronal ADC, b value = 1000 | 1.66 ± 5.36 | 1.07 ± 0.64 | 0.35 ± 0.13 | 0.25 |
| Coronal ADC, b value = 3000 | 1.34 ± 3.87 | 0.97 ± 0.60 | 0.28 ± 0.15 | 0.27 |
| Coronal ADC, b value = 5000 | 1.03 ± 1.76 | 1.09 ± 0.72 | 0.21 ± 0.11 | 0.09 |
A Comparison of ADC values in Various Hormone-Secreting Types of Pituitary Adenomas
Table 3 shows the relative SI and ADC of tumors in comparison with normal white matter of temporal lobe in each consistency group. The difference in axial and coronal relative SI in T1- and T2-weighted images and axial and coronal relative ADC value with different b values (0, 1000, 3000, and 5000) between groups was not significant.
| SI of Tumor to SI of Normal White Matter | Tumor Consistency | P Value | ||
|---|---|---|---|---|
| Soft (N = 12) | Intermediate (N = 29) | Hard (N = 3) | ||
| Axial T1-weighted image | 1.01 ± 0.52 | 0.83 ± 0.42 | 0.67 ± 0.29 | 0.44 |
| Axial T2-weighted image | 4.79 ± 1.97 | 1.30 ± 0.79 | 0.97 ± 0.54 | 0.49 |
| Coronal T1-weighted image | 0.74 ± 0.39 | 0.49 ± 0.28 | 0.41 ± 0.15 | 0.09 |
| Coronal T2-weighted image | 1.17 ± 0.82 | 0.86 ± 0.51 | 0.70 ± 0.46 | 0.47 |
| Axial ADC, b value = 0 | 1.65 ± 1.27 | 4.15 ± 11.30 | 0.82 ± 0.27 | 0.57 |
| Axial ADC, b value = 1000 | 1.53 ± 1.09 | 3.52 ± 10.01 | 0.68 ± 0.44 | 0.42 |
| Axial ADC, b value = 3000 | 1.36 ± 0.90 | 2.52 ± 6.81 | 0.60 ± 0.37 | 0.35 |
| Axial ADC, b value = 5000 | 1.50 ± 0.98 | 2.11 ± 4.07 | 0.73 ± 0.50 | 0.37 |
| Coronal ADC, b value = 0 | 1.17 ± 0.92 | 5.19 ± 23.59 | 0.58 ± 0.27 | 0.24 |
| Coronal ADC, b value = 1000 | 1.04 ± 0.83 | 1.85 ± 6.10 | 0.53 ± 0.13 | 0.24 |
| Coronal ADC, b value = 3000 | 0.98 ± 0.83 | 1.41 ± 4.36 | 0.53 ± 0.12 | 0.51 |
| Coronal ADC, b value = 5000 | 1.04 ± 0.91 | 1.02 ± 1.96 | 0.60 ± 0.16 | 0.64 |
Conventional MRI Findings and ADC Values in Pituitary Adenomas with Different Consistencies
In the next step, we investigated the correlation of tumor consistency and ADC values with collagen content of tumors. 42 out of 45 adenomas had collagen staining available for analyses. Among 11 soft tumors, five (45.5%) had mild and six (54.5%) had moderate collagen content. Twenty-eight tumors with intermediate consistency revealed mild collagen content in 9 (32.1%), moderate in 12 (42.9%), and high in 7 (25%) patients. In the hard consistency group, 2 (66.7%) tumors had mild and one (33.3%) had a high collagen content. The collagen content showed a significant diversity among all groups (P value = 0.05). Here again, no significant differences were found between the mean SI and ADC values with regard to the collagen content among the three groups (Table 4).
| SI of Tumor to SI of Normal White Matter | Collagen Content | P Value | ||
|---|---|---|---|---|
| Mild (N = 14) | Moderate (N = 20) | Severe (N = 8) | ||
| Axial T1-weighted image | 0.89 ± 0.47 | 0.96 ± 0.46 | 0.67 ± 0.25 | 0.28 |
| Axial T2-weighted image | 1.22 ± 0.56 | 3.46 ± 0.27 | 1.34 ± 1.02 | 0.60 |
| Coronal T1-weighted image | 0.63 ± 0.47 | 0.55 ± 0.26 | 0.41 ± 0.16 | 0.51 |
| Coronal T2-weighted image | 0.91 ± 0.69 | 1.00 ± 0.63 | 0.79 ± 0.35 | 0.85 |
| Axial ADC, b value = 0 | 6.44 ± 17.05 | 1.48 ± 1.37 | 3.44 ± 5.52 | 0.90 |
| Axial ADC, b value = 1000 | 5.67 ± 15.36 | 1.35 ± 1.07 | 2.58 ± 3.56 | 0.92 |
| Axial ADC, b value = 3000 | 4.09 ± 10.57 | 1.29 ± 0.91 | 1.38 ± 1.35 | 0.92 |
| Axial ADC, b value = 5000 | 3.19 ± 6.48 | 1.30 ± 0.94 | 1.57 ± 1.29 | 0.77 |
| Coronal ADC, b value = 0 | 10.75 ± 35.19 | 0.79 ± 0.57 | 0.83 ± 0.42 | 0.75 |
| Coronal ADC, b value = 1000 | 3.28 ± 8.75 | 0.71 ± 0.49 | 0.65 ± 0.35 | 0.90 |
| Coronal ADC, b value = 3000 | 2.60 ± 6.48 | 0.66 ± 0.42 | 0.61 ± 0.27 | 0.89 |
| Coronal ADC, b value = 5000 | 0.92 ± 0.88 | 1.20 ± 2.38 | 0.66 ± 0.34 | 0.85 |
Conventional MRI Findings and ADC Values in Tumors with Different Collagen Content
We examined 45 cases of macroadenoma and found that neither the tumor consistency nor the collagen content was correlated with the ADC values or the tumor appearance on conventional MRI.
4. Discussion
The success of pituitary macroadenoma surgery depends on the size, location, invasiveness, and consistency of the tumor. Prediction of tumor consistency would be a helpful method to plan an appropriate surgical procedure and technique. Radiological imaging of the sellar region is a challenging task. Conventional pre- and post-gadolinium enhanced T1-weighted and T2-weighted sequences provide a qualified image of the normal pituitary gland, as well as size, location, and invasiveness of an adenoma. DWI findings based on water diffusion within the tissue may provide information about tumor consistency. The application of DWI technique for the evaluation of pituitary macroadenomas is a rare approach and published results are controversial.
In this study, we assessed the predictive value of both conventional MRI and DWI techniques for tumor consistency in 45 macroadenomas while considering both surgical and pathological findings. As previously reported, we found that the density of the collagen content of tumor specimens was correlated with tumor consistency (3, 11). The collagen content of the hard tumors was significantly higher than that of the tumors with soft and intermediate consistency. However, tumor consistency and collagen content had no correlation with either SI or ADC values on MR imaging.
Previous studies assessed the correlation of conventional MRI findings with macroadenoma consistency, by focusing on T2-weighted signal intensity, reporting controversial results. Snow et al., Iuchi et al., and Ishii et al. demonstrated that fibrous tumors had lower signal intensity (2, 7, 8, 13), whereas others revealed no correlation (3, 9, 10); and even one study showed inverse correlation between tumor consistency and SI on conventional MR images (14). We found no significant difference between tumor to normal white matter T2-weighted signal intensity and tumor consistency during surgery. Authors suggested that this discrepancy among results could be due to the effect of various factors on the T2-weighted signal intensity including tumor cellularity, nuclear to cytoplasmic ratio, free water in the extracellular space, and cystic component in the tumor (14, 15). High cellularity with a high nuclear to cytoplasm ratio and high collagen content can decrease signal intensity, whereas high extracellular free water increases SI on T2-weighted images (16). Thus, T2-weighted images seem to have limited values in predicting tumor consistency.
Pierallini et al. demonstrated that adenomas with higher consistency displayed higher ADCs, whereas adenomas with a soft consistency showed higher cellularity and lower ADC values (14). They concluded that the ADC was positively correlated with the hardness of tumor. Moreover, they suggested that tumors with ADC values greater than 1.0 × 10-3 at b = 1000 were difficult to aspirate. Instead, Suzuki et al. found no relationship between tumor consistency and ADCs (b = 1000) of soft and intermediate macroadenomas (16).
Boxerman et al. found that adenomas with ADC ratios greater than 1.1 (b = 1000) were highly resectable. Furthermore, the ADC ratios in reticulin-poor tumors were significantly higher (15).
The correlation between tumor consistency and ADC values using periodically rotated overlapping parallel lines with enhanced reconstruction (PROPELLER) DWI technique was evaluated by Mahmoud et al. They concluded that ADC was not correlated with the consistency and hormone-secretion status of pituitary adenomas (17).
On the other hand, a positive correlation between the mean ADC value and tumor consistency was found by Mahamed et al. and they recommended a cut-off value of 0.6 × 10-3 mm2/s (b = 1000) for discrimination of the intermediate/soft macroadenomas from hard adenomas (18).
In this study, we had a larger sample size (N = 45) and calculated ADC in various b values (0, 1000, 3000, and 5000). We found that tumors with hard consistency display a slightly lower T2-weighted SI and ADC in different b values compared to intermediate and soft tumors, although it was not appreciably significant. Our results are consistent with Suzuki et al. and Mahmoud et al. findings that indicate DW and conventional MR imaging cannot provide sufficient information about predicting macroadenoma consistency (16, 17). However, our findings were not in line with those of Pierallini et al. and Mohamed et al. (14, 18). They showed that higher cellularity and scant fibrous stroma of soft tumors can lead to lower ADC values and estimated a cut-off value for prediction of tumor consistency. In the present study, we encountered soft tumors with the mean ADC values higher than the cut-off value estimated in the mentioned study and in contrast to their results, they were easily suctioned at surgery. Other studies suggested that the ADC is also affected by extracellular volume fraction (19). It has been proposed that the higher collagen density in the extracellular space of hard tumors could decrease diffusion and result in lower ADC values (20).
Previous studies showed that GH-producing adenomas manifest significantly lower SI on T2-weighted images due to high protein concentration inside the secretory granules (10, 17). We observed no significant difference in the conventional and DW MR imaging indices among pituitary adenomas with different secretory functions.
Limitation of our study was the lack of quantitative analysis of collagen content. Due to piecemeal resection of adenomas during surgery, samples represent only a small fraction of the entire tumoral tissue. Qualitative assessment of collagen is a highly subjective method. Therefore, quantitative analysis of collagen content may help make grading system less subjective. Moreover, the number of hard tumors in our study was small (N = 3). Further studies on larger sample sizes are recommended to evaluate the potential use of ADC in the preoperative assessment of pituitary adenomas.
4.1. Conclusion
Conventional MRI measures and ADCs at different b values and different planes are not correlated with the consistency, collagen content, and hormone-secreting status of pituitary macroadenomas. Preoperative prediction of tumor consistency cannot be reliably obtained with DWI techniques.