1. Background
Methamphetamine (MA) (Meth/MA; Crystal; Chalk, or Ice; C10H15N) is a frequently abused drug with a remarkable potency profile and pronounced addictive potential (1). Methamphetamine elevates energy levels, mood, and attention in the short term and has received approval for therapeutic use in treating obesity, narcolepsy, and Attention-Deficit Hyperactivity Disorder (ADHD) (2). All amphetamine-type agents stimulate the Central Nervous System (CNS), primarily by altering various components of neurotransmitter networks. These include the blockade and reversal of vesicular monoamine and dopamine transporters, inhibition of monoamine oxidase, facilitation of catecholamine release, and modification of monoamines-glutamate/GABA interactions (3). Additionally, less recognized molecular pathways, beyond the direct transmitter-mediated ones, strongly contribute to the effects of MA (4, 5).
Although these neurochemical effects predominantly occur in reward and executive-related brain areas (6, 7), MA also shifts the overall brain signal-to-noise ratio, potentially leading to both beneficial and adverse outcomes (8). Current research indicates that MA has a wide range of effects on neurocognitive performance, from enhancing certain aspects of attentional processing (9) to having no effect (7, 10-13) or even causing significant cognitive disruptions and other adverse outcomes (14). The nature, onset, persistence, sequence, and reversibility of these neurobehavioral effects are still only partly understood (e.g., working memory deficits are evident immediately and may persist after abstinence) (15).
Multiple lines of evidence support potential improvements in psychomotor functioning, perceptual speed (inspection time), vigilance (both accuracy and speed), and tracking ability on divided attention tasks following MA exposure (16-24). While low doses of MA may lead to mild cognitive decline, memory deficits, poor inhibitory control, and anxiety, the most severe effects of high doses include severe depressive or psychotic episodes, violent behaviors, and intractable seizures (25-27). Kennedy et al. reported mixed results; they examined the effects of 10 mg of d-amphetamine on a battery of cognitive tests and found improved performance on most tasks, but a decline in visual search abilities due to sympathetic arousal and perceptual narrowing (28).
In a medical context, patients typically receive lower doses of MA than those abused by individuals. For example, the dose for long-term management of ADHD ranges from 5 to 30 mg/kg, whereas a single dose of crystal meth for a "rush" can be approximately 40 - 60 mg. Although the rush lasts only a few minutes, the resulting euphoria sustains addictive behavior, leading users to increase the frequency (known as a "run") or amount of MA, or even engage in binge dosing to reexperience the rush. Given the high risk of methamphetamine abuse and the potential for extensive CNS damage, physicians rarely prescribe it for medical conditions.
Methamphetamine induces a complex neuropathology network involving disorganized monoaminergic systems, excitotoxicity, and neuroinflammation (29-31). Chronic exposure leads to neurotoxicity, resulting in structural and functional impairments in neurons and behaviors. Damage to dopaminergic and serotonergic terminals occurs due to dopamine release, oxidative stress, inflammation, and neuronal death. Glutamate also plays a role in MA-induced cytotoxicity, inflammation, and cell death by affecting NMDA signaling and synaptic proteins. NMDA-evoked dopamine release and PI3/Akt phosphorylation activate NF-κB, which promotes inflammation, neurotoxicity, and apoptosis. Inflammatory responses are proposed as the final common pathway in MA pathology (32).
Early studies focused on microgliosis and astrogliosis as potential mechanisms (33). More recent research has highlighted the role of inflammasomes, particularly the NLRP3 inflammasome, in triggering inflammation (34, 35). Activation of NLRP3 by damage-associated molecular patterns (DAMPs) leads to caspase-1 activation and subsequent inflammation. Methamphetamine exposure triggers ASC aggregation and damages lysosomes and mitochondria. The activated inflammasome contributes to chronic CNS inflammation and secondary neuronal damage, leading to pyroptosis, a distinct form of cell death (36-38). Although the exact mechanism of MA-induced neuroinflammation remains unclear, evidence suggests the involvement of innate immune cells and microglia (34, 39).
Previous studies have shown inconsistent results (null, beneficial, and adverse) regarding the impact of chronic administration of low-to-moderate doses of MA on neurocognition, and the extent to which these effects are attributable to neuroinflammatory processes. However, as the growing body of literature indicates the significant neurotoxicity of higher addictive doses, some research suggests that even small quantities of crystal meth may trigger toxic cascades through yet undefined pathways (31, 40, 41). Given the medical approval of MA and its implications for public health, the unclear mechanisms of neuroinflammation and the broad range of potential outcomes necessitate a critical evaluation of timing and chronological variables on MA-induced effects.
To address these gaps, we designed a study to assess hippocampal inflammation and explore its functional consequences under a subchronic, low-dose regime (5 mg/kg) (42) for 1, 2, and 3 weeks.
2. Objective
The study focused on the structure of the hippocampus, specifically the CA1 region—known for containing the major output relay neurons and being one of the most vulnerable subfields within the hippocampus—the activation of NLRP3 inflammasomes, and the performance of spatial, passive avoidance, and recognition memory tasks.
3. Methods
3.1. Grouping
A total of 126 healthy male Wistar rats, each weighing between 260 - 300 g, were used in this study. The rats were acclimatized for one week in a controlled environment with appropriate temperature, lighting, and access to standard food and water. The study was structured into three primary groups based on the duration of MA administration: One week, 2 weeks, and 3 weeks. Each primary group was further divided into two subgroups: One receiving daily intraperitoneal injections of MA (5 mg/kg) and the other receiving an equivalent volume of normal saline (0.2 mL). This design resulted in six groups in total, with 21 rats per group.
Each subgroup was further divided based on the type of memory tasks used, with 7 rats allocated for each task (spatial memory, novel object recognition, and passive avoidance). After completing the memory (cognitive) tests at the respective administration periods of 1, 2, or 3 weeks, the 7 rats from each task group were divided again: Four rats were used for gene expression analysis and 3 rats for histological analysis. This allocation ensured sufficient sample sizes for both types of analyses. The study protocol was approved by the TUMS Animal Care and Use Committee (Ethical code: IR.TUMS.MEDICINE.REC.1399.949).
3.2. Y-maze
The Y-maze is a closed maze consisting of three arms arranged in a "Y" shape, commonly used to assess spatial working memory in rodents (43). It is a valuable tool for studying various cognitive functions under different conditions, such as brain lesions, diseases, chronic stress, and aging. In this study, rats were allowed to explore the maze to observe their ability to remember previously visited arms and alternate their choices. The researchers recorded the rats' arm entries, specific arm choices, and sequential alternations. Spatial learning was evaluated using the "spontaneous alternation" protocol, and the spontaneous alternation behavior (AB) and activity level (AL) were calculated using the following formulas:
Alternation Behavior (AB) (%) = Correct Sequence (CS) / Correct Sequence (CS) + Incorrect Sequence (IS) × 100%
Activity Level (AL) = CS + IS + 2
In the Y-maze task, CS (Correct Sequence) represents when a rat enters a different arm in each of three consecutive arm entries, indicating successful spatial memory and alternation behavior. IS (Incorrect Sequence) refers to when a rat repeatedly enters the same arm that was previously visited within the three consecutive arm entries, indicating a failure to alternate and a potential deficit in spatial memory.
3.3. Novel Object Recognition Test
The Novel Object Recognition (NOR) test (44) is conducted in a plexiglass box with high walls (40 × 40 × 38 cm). During the test, animals are first habituated to the environment for 3 minutes, followed by a 10-minute exploration period with two identical objects. After a 1-hour interval, one of the objects is replaced with a new one, and the animals are expected to spend more time investigating the novel object. This test evaluates non-spatial learning of object identity, engaging brain regions such as the hippocampus and prefrontal cortex.
Novelty preference (NP %) = Tnovel/ Tfamiliar + Tnovel × 100%
Discrimination Index (DI) = Tnovel – Tfamiliar/ Tfamiliar + Tnovel
Thus, the Novel preference (NP) score ranged from 0% (indicating no exploration of the novel object) to 100% (indicating exclusive exploration of the novel object), while the Discrimination Index (DI) ranged from -1 (indicating exclusive exploration of the familiar object) to +1 (indicating exclusive exploration of the novel object).
3.4. Passive Avoidance
The passive avoidance (PA) experiment (45) involved two compartments—an illuminated compartment (IC) and a dark compartment (DC) separated by a door. Rats were given 5 minutes to explore both compartments, followed by three 5-minute trials with a 30-minute break between each trial. During the third trial, a foot shock (50 Hz, 1.5 mA, for 1 second) was administered to the rat upon entering the DC. After 10 seconds of confinement, the rat was returned to its home cage. A memory retention test was conducted 24 hours later, during which the time taken (step-through latency, STL) for the rat to enter the DC for the first time was measured. Rats with intact memory typically avoid the DC due to the association with the foot shock. This paradigm assesses associative learning and involves brain regions such as the amygdala, hippocampus, frontal cortex, and cingulate cortex.
3.5. Quantitative Real-time PCR (qRT-PCR)
After the memory test, four rats from each group were euthanized, and their hippocampi (from both hemispheres) were quickly frozen and stored at -70°C. Total RNA was extracted using QIAzol Lysis reagent, and its quality and quantity were assessed using a NanoDrop™ spectrophotometer. Although gel electrophoresis is a valuable technique for determining RNA quality, the NanoDrop™ spectrophotometer was chosen for its rapidity and efficiency in quantifying nucleic acids, particularly with small sample volumes. The quality of the extracted RNA was further confirmed through subsequent RT-PCR analysis.
cDNA was synthesized from 1 μg of RNA using the RevertAid First Strand cDNA Synthesis Kit (Thermo Fisher Scientific). Real-time PCR was then performed to measure the expression levels of NLRP3, ASC, and Caspase-1 mRNA genes using the StepOnePlus Real-Time PCR System (Thermo Fisher Scientific). For gene amplification, we used the MasterMix SYBR Green from the BioFact brand without ROX, which contains dNTPs, buffer, Taq polymerase, and SYBR Green dye for fluorescence detection.
The annealing temperatures and NCBI codes for gene blasting were determined using the Primer-BLAST tool on the NCBI website. The Rotor-Gene Q real-time PCR machine (Qiagen) was used for the real-time PCR analysis. During each cycle of amplification, the fluorescence signal emitted by SYBR Green dye bound to double-stranded DNA was monitored to quantify the PCR product in real-time. The threshold cycle (Ct) values obtained were used to calculate the relative expression levels of the NLRP3, ASC, and Caspase-1 genes.
To ensure accuracy and consistency in our data, the expression levels of target genes were normalized to the reference gene B2M (Beta-2-Microglobulin), chosen for its stable expression across samples. This normalization helped correct for variations in RNA input amounts and overall PCR efficiency. The resulting melting curves from the RT-PCR analysis indicated the presence of the intended genes. We conducted our processes with corresponding negative controls, and the associated melting curves further confirmed the specificity and accuracy of our procedure and the genetic material obtained. Details of the primers, including annealing temperatures and NCBI codes, are provided in Table 1.
| Genes and Primer | NCBI Codes | Annealing Tm (°C) | Product Size (bp) | Tm Melt (̊C) |
|---|---|---|---|---|
| B2M (Reference) | NM_004048 | 60 | 151 | 82.8 |
| Forward: CTTTCTACATCCTGGCTCACAC | ||||
| Reverse: GTCCAGATGATTCAGAGCTCC | ||||
| CASP1 | NM_033292 | 60 | 209 | 86.1 |
| Forward: CCACTCGTACACGTCTTGC | ||||
| Reverse: GTCAGAAGTCTTGTGCTCGG | ||||
| ASC | NM_013258 | 60 | 181 | 83.9 |
| Forward: TCTGGAGGGGTATGGCTTGG | ||||
| Reverse: GAGTGCTTGCCTGTGTTGGT | ||||
| NLRP | NM_004895 | 60 | 196 | 83.2 |
| Forward: CTGACCCATAACCAGAGCCTCC | ||||
| Reverse: CAGTCAGCTCAGGCTTTTCCTC |
3.6. Histology
In the histology procedure, three rats were administered ketamine and xylazine (80 mg/kg and 10 mg/kg, respectively) to achieve deep anesthesia. The brains were then perfused with 150 mL of phosphate buffer solution (PBS) (0.1M, pH 7.4), followed by 4% paraformaldehyde (PFA) via the intra-cardiac route. After perfusion, the rats' heads were decapitated using a guillotine, and the entire brains were meticulously extracted from the skulls and prepared for Nissl staining.
3.7. Statistical Analysis
Data were expressed as means ± standard error of the mean (SEM). Appropriate parametric tests, including one-way and two-way ANOVA, were used for the analysis, followed by post-hoc tests. A value of P < 0.05 was considered statistically significant. Data analysis was performed using GraphPad Prism version 10 software.
4. Results
4.1. Assessment of Spatial Working and Recognition Memory Using the Y-Maze Test
4.1.1. Alternation Behavior and Activity Level
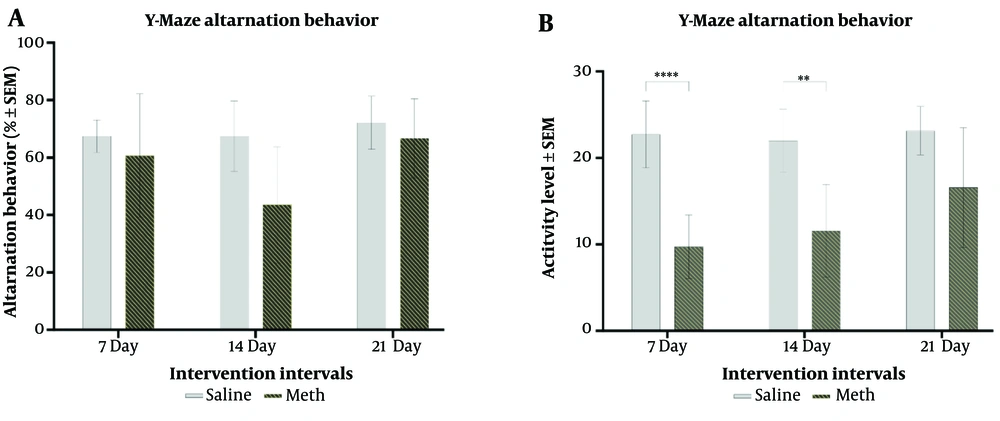
Figure 1 illustrates a significant decrease in spontaneous alternation behavior after two weeks of methamphetamine treatment compared to controls (F (1,36) = 6.819, P = 0.013). However, the difference observed after three weeks of treatment was not statistically significant. In contrast, activity level significantly increased in the MA group after 1 week (P = 0.000) and 2 weeks (P = 0.0018) of treatment, with a progressive increase from 1 to 3 weeks (F (1,36) = 50.06, P = 0.0001). Despite this trend, the difference in AL between 7 days and three weeks of treatment was not significant.
A, effects of daily methamphetamine (5 mg/Kg) or saline (200 µL) administration for 1, 2, and 3 weeks on the spontaneous alternation behavior in Y-Maze; B, effects of daily methamphetamine (5 mg/kg) or saline (200 µL) administration for 1, 2, and 3 weeks on the rat’s activity level in Y-Maze (depicted as counts) (Mean ± SEM of 7 rats per group. ** P ≤ 0.01; **** P ≤ 0.0001, 2-Way ANOVA).
4.2. Assessment of Object Recognition Memory Using the Novel Object Recognition Test
4.2.1. Assessment of Object Exploration Behavior
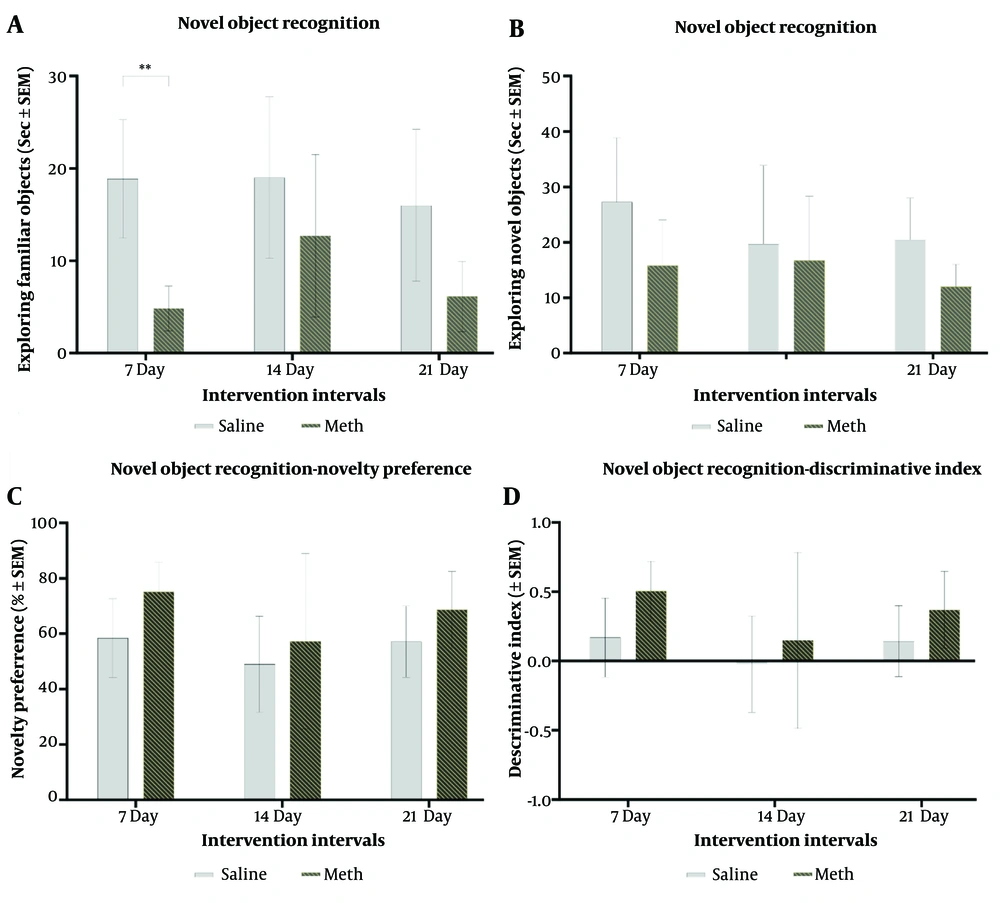
Based on the first type of assessment, familiarity detection initially increased but then decreased after 21 days of methamphetamine treatment. The type of intervention significantly affected familiar object detection (F (1,36) = 22.50, P = 0.0001). For novelty detection scores, treatment type was significant (F (1,36) = 6.017, P = 0.019), but neither time nor the interaction between treatment and time showed significance. Meth-treated groups generally spent more time exploring the novel object compared to controls (F (1,36) = 4.74, P = 0.36), indicating a trend, although not statistically significant, toward increased novelty preference. Temporal duration did not significantly affect novelty preference. While pairwise comparisons did not reach statistical significance, meth-treated groups demonstrated improved discrimination ratios compared to controls (F (1,36) = 4.75, P = 0.035) (Figure 2).
A, effects of daily methamphetamine (5 mg/kg) or saline (200 µL) administration for 1, 2, and 3 weeks on the time spent exploring familiar objects in the Novel Object Recognition test; B, effects of daily methamphetamine (5 mg/kg) or saline (200 µL) administration for 1, 2, and 3 weeks on the time spent exploring novel objects in the Novel Object Recognition test; C, effects of daily methamphetamine (5 mg/kg) or saline (200 µL) administration for 1, 2, and 3 weeks on the rat’s novelty preference in the Novel Object Recognition test; D, effects of daily methamphetamine (5 mg/kg) or saline (200 µL) administration for 1, 2, and 3 weeks on the rat’s discriminatory behavior between the novel (B) and familiar (A) object in the Novel Object Recognition test (Mean ± SEM of 7 rats per group. ** P ≤ 0.01, 1-Way ANOVA).
4.3. Assessment of Fear-Conditioning Memory using the Passive Avoidance Test
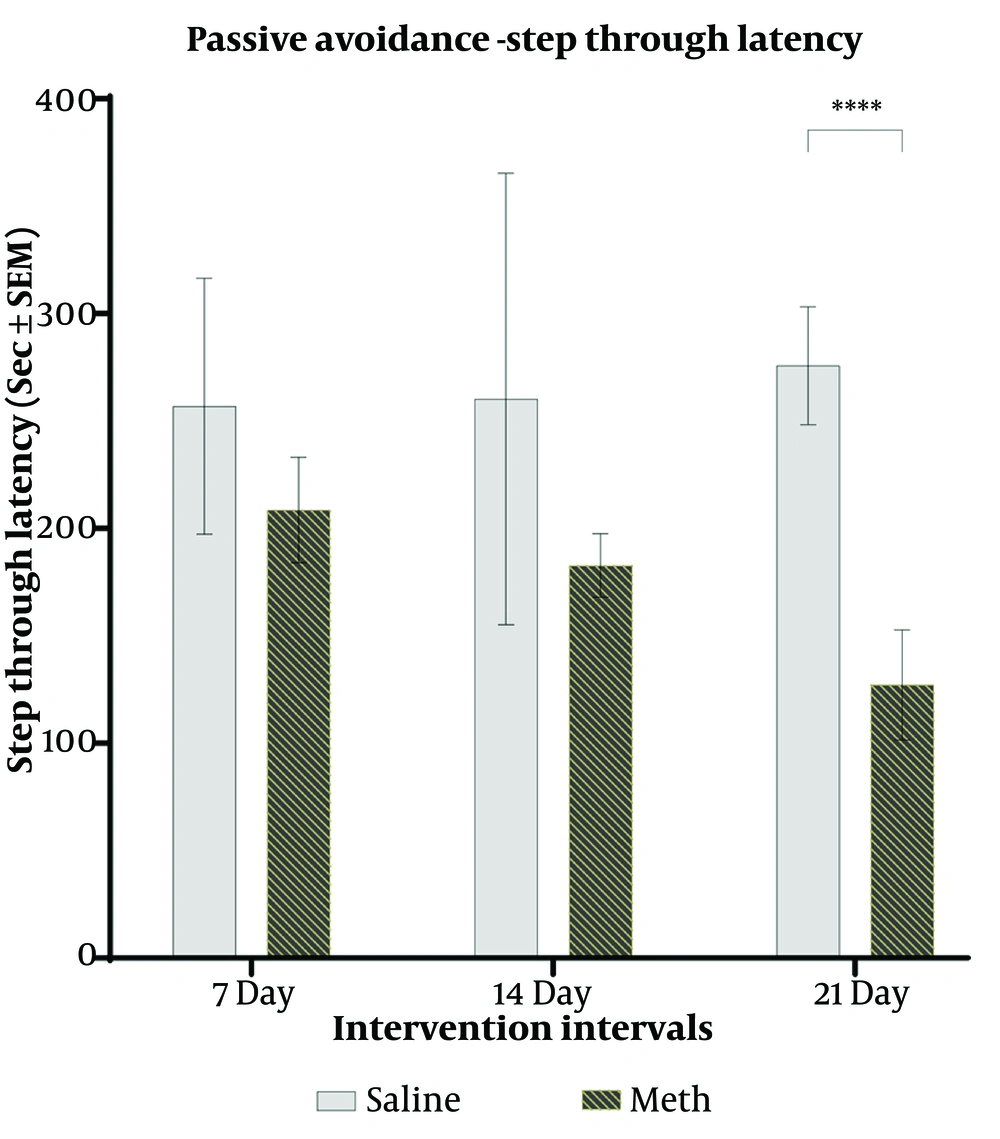
Normal rats in the control group, with intact memory, were reluctant to enter the dark compartment (DC). The current results showed a significant interaction effect between time and type of intervention (F (1,36) = 3.319, P = 0.047). However, no significant difference in step-through latency (STL) was observed between the control and methamphetamine (meth) groups after 1 and 2 weeks of meth treatment. Nevertheless, there was a significant decrease in STL in the meth group after 3 weeks of treatment compared to their controls (F (1,36) = 31.33, P = 0.0001) (Figure 3).
4.4. Assessment of NLRP3, ASC, and Caspase-1 Genes Expressions Using the Quantitative Real-time Polymerase Chain Reaction
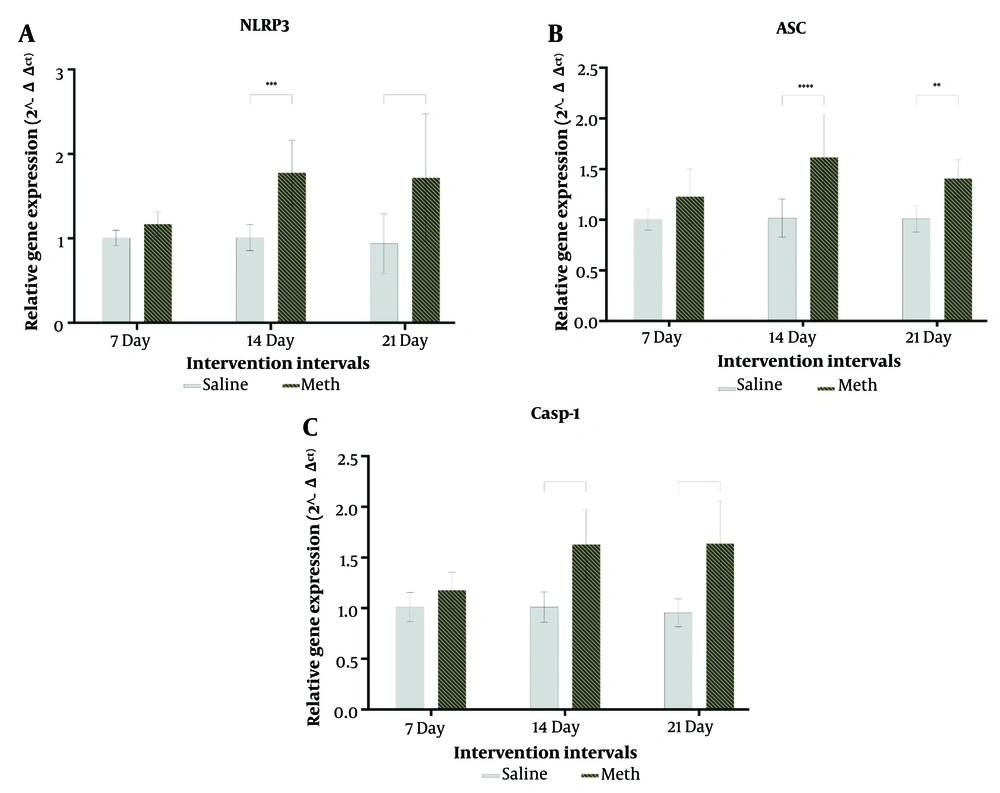
The expressions of NLRP3 (P = 0.9017), ASC (P = 0.2191), and caspase-1 (P = 0.6080) genes in rats treated with methamphetamine for one week were not significantly different from their controls. However, there were significant increases in the expressions of NLRP3, ASC, and caspase-1 genes in rats treated with MA for two weeks (NLRP3 P = 0.0001, ASC P = 0.0001, Caspase-1 P = 0.0001) and three weeks (NLRP3 P = 0.0001, ASC P = 0.008, Caspase-1 P = 0.0001) compared to their respective controls (Figure 4).
A, effects of daily methamphetamine (5 mg/kg) or saline (200 µL) administration for 1, 2, and 3 weeks on the NLRP3 mRNA gene expression; B, effects of daily methamphetamine (5 mg/kg) or saline (200 µL) administration for 1, 2, and 3 weeks on the ASC mRNA gene expression; C, effects of daily methamphetamine (5 mg/kg) or saline (200 µL) administration for 1, 2, and 3 weeks on the caspase-1 mRNA gene expression (mean ± SEM, N = 21, ** P ≤ 0.01; *** P ≤ 0.001; **** P ≤ 0.0001, unpaired t-test).
4.5. Histology
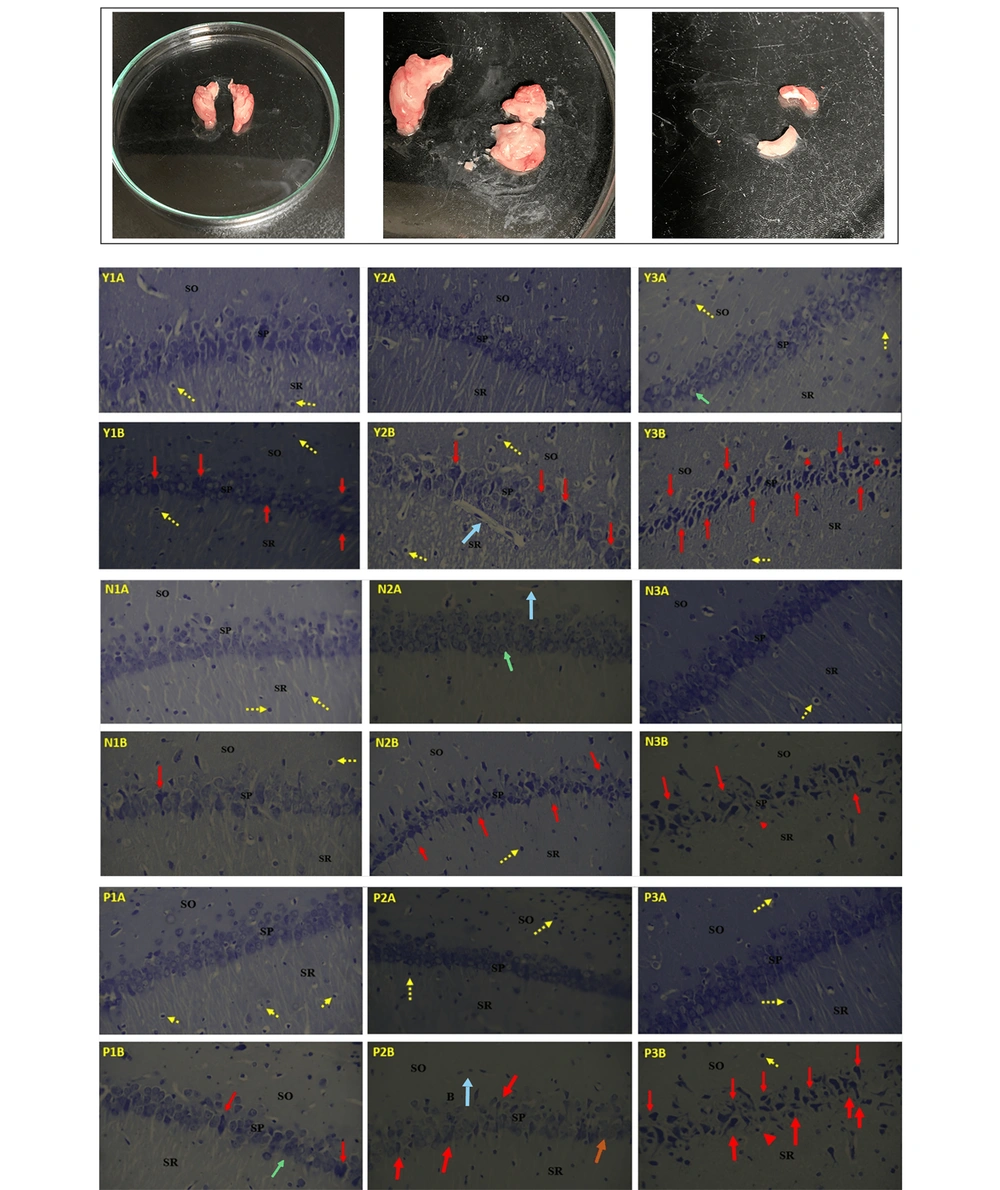
Plate I display photomicrographs of the CA1 region of the hippocampi of Wistar rats, highlighting the stratum oriens (SO), stratum pyramidale (SP), and stratum radiatum (SR). Slides 1A, 2A, and 3A represent the control groups for the Y-maze (Y), Novel Object Recognition (N), and Passive Avoidance (P) tests, respectively, which received 0.2 mL of 0.9% normal saline for 1, 2, and 3 weeks. All control slides (1A, 2A, and 3A) exhibit well-arranged, densely packed pyramidal cell layers with normal histoarchitecture of the hippocampus. The neuronal cytoplasm in the pyramidal cells is clearly visible, displaying both euchromatic and heterochromatic stages. Traces of neuronal fibers are visible in the SR, interspersed with microglial cells (yellow arrows) and blood vessels (yellow arrowhead).
Slides 1B, 2B, and 3B correspond to rats treated with 5 mg/kg of methamphetamine (MA) for 1, 2, and 3 weeks. In Y1B, N1B, and P1B, the pyramidal cell layer is visible but slightly disorganized, with a few degenerating cells (red arrows) and less distinct neuronal cytoplasm (black arrows). The SR shows traces of slightly broken neuronal fibers, and the SO contains some neuroglial supporting cells.
In Y2B, N2B, and P2B, numerous degenerating and degenerated pyramidal cells (red arrows) are observed. The neuronal fibers in the SR are virtually absent, and the SO contains few supporting neuroglial cells.
In Y3B, N3B, and P3B, most pyramidal cells appear shrunken, degenerated (red arrows), and pyknotic, with a few vacuolated cells (red arrowhead). Neuronal fibers are completely absent in the SR, and the SO contains pyknotic (Py) and necrotic neuroglial supporting cells devoid of blood vessels (Plate I) (Figure 5).
The isolated hippocampus tissue and light micrographs of different layers (SO, SP, SR) of the hippocampus. The most upper row shows the different plates of hippocampus sections (not specified for each condition). Control groups (1A, 2A, 3A) were administered normal saline for 1, 2, and 3 weeks respectively; while the experimental groups (1B, 2B, 3B) were administered methamphetamine for 1, 2, and 3 weeks respectively. Y-maze control (Y1A, Y2A, Y3A) and meth groups (Y1B, Y2B, Y3B). Novel object recognition control (N1A, N2A, N3A) and meth groups (N1B, N2B, N3B). Passive avoidance control (P1A, P2A, P3A) and meth groups (P1B, P2B, P3B). SO, stratum oriens; SP, stratum pyramidal; SR, stratum radiatum, yellow arrows = microglial cells, blue arrow = blood vessels, green arrows=neuronal cytoplasm, red arrows = degenerating/ degenerated cells, red arrowhead = vacuolated cells. Nissl stain, magnification x400.
5. Discussion
Neurotoxicity has been observed in various studies involving different dosing regimens of methamphetamine, and it is well established that multiple areas of the brain can be affected by these toxic pathways. However, the effects of subchronic low doses of MA on the brain were not well understood. In this study, we demonstrated that even lower doses of MA can be neurotoxic through the activation of the inflammasome complex in the CA1 region of the hippocampus and through specific dimensions of neurocognitive functioning.
Neurodegeneration observed in pyramidal cells increased with the duration of MA treatment, leading to the complete destruction of hippocampal histoarchitecture after three weeks. Methamphetamine triggers hippocampal gliosis, cytokine production, and significant alterations in cytoskeletal, synaptic, and axonal proteins, resulting in massive cell death via necrosis (46), apoptosis (47, 48), and pyroptosis (49-51). The hippocampus is particularly vulnerable due to its intrinsic cytoarchitecture and barriers. Liquid chromatography-mass spectrometry analysis revealed significant protein modifications, particularly in the CA1 region, making it the most vulnerable area to MA. Factors such as glutamate-calcium-mediated cytotoxicity, plasticity, and alteration of the blood-brain barrier (BBB) contribute to hippocampal vulnerability (52-54). Methamphetamine-induced BBB permeability increases transiently, predominantly affecting the hippocampus. Galectin-1 expression in endothelial cells may mitigate the enhancement of BBB permeability (55). The nature of these mechanisms implies that consumption patterns can fluctuate or even reverse observed manifestations over time.
Low doses of MA (5 mg/kg) administered for two or three weeks were found to activate the NLRP3-ASC-caspase-1 inflammasome response, challenging previous beliefs. This activation correlated with cognitive changes and neuroinflammatory pathways enriched in MA-induced neurotoxicity. Activation of caspase-1 leads to pyroptosis, a highly inflammatory form of cell death. The increased expression of caspase-1 mRNA after MA administration likely induced neuroinflammation and pyroptosis in the hippocampi (34). Additionally, neuroimmune interactions in the hippocampus contribute to homeostatic balance, plasticity, and resilience mechanisms. Methamphetamine injection also activated growth-associated signaling pathways, leading to inflammation via glial cell reactivation in the striatum and hippocampus. This inflammation persisted for up to three weeks and could contribute to increased rewarding responses (35, 56).
Our study revealed that daily administration of 5 mg/kg of methamphetamine for two weeks impairs spatial memory compared to the saline group. However, this difference in spatial processing was not significant after one or three weeks of MA or saline administration, which may be attributed to the duration of MA exposure. One week of treatment may have been insufficient to induce the significant neurotoxicity or neurodegeneration necessary to impair memory. Previous studies have provided conflicting results regarding the effects of MA on memory, with some showing improved performance after one week of administration and deficits following prolonged abstinence, while others have reported deficits after specific treatment regimens.
The CA1 region of the hippocampus integrates information from the CA3 region and the entorhinal cortex, enabling match-mismatch detection and error processing, and plays a crucial role in encoding and retrieving spatial information. Functional and structural neuroimaging studies have highlighted the distinct roles of hippocampal subfields in cognitive functioning, with the dentate gyrus and CA3 primarily involved in pattern recognition and early retrieval, and the CA1 in consolidation, late retrieval, and spatial encoding. The three-week MA treatment may have led to tolerance or remodeling of neurotoxic effects, similar to observations in methamphetamine addicts reported by other researchers (57).
Methamphetamine treatment affected fear response memory only after three weeks of exposure, consistent with previous studies reporting impaired passive avoidance memory with both higher and lower doses of MA. The decreased step-through latency in MA-treated rats may be partly explained by the analgesic effects of MA and hippocampal injuries following the three-week treatment course (58). Dopamine, sourced from areas such as the locus coeruleus and ventral tegmental area, plays a critical role in spatial learning, memory formation, and contextual fear conditioning in the hippocampus. Irregularities in the dopamine-related WNT signaling pathway are also implicated in motor learning and reward-associated memories in response to MA (56). Studies have shown that MA can deplete dopamine levels in both the anterior and posterior striatum, with anterior striatal dopamine levels predicting passive avoidance performance (32). Therefore, the delayed destructive effects of methamphetamine on behavior may be due to the relative resistance of the striatum compared to the hippocampus.
One concern regarding the current findings is that other areas of the brain were not assessed, despite the possibility that these areas might also play direct or indirect roles in cognition and inflammatory responses to MA (59). Further analyses are needed to clarify these aspects. Another major limitation is the lack of measurement of other inflammatory parameters, as inflammatory responses are highly interconnected. Assessing molecular markers related to the general immune state and apoptosis/pyroptosis-related pathways could have provided more precise and comprehensive interpretations. Additionally, the study's approach is limited by the timing of assessments; longer temporal durations are needed to capture truly chronic patterns.
5.1. Conclusions
In conclusion, this study found that even lower therapeutic doses of methamphetamine can cause neurotoxicity in the hippocampus, particularly in the CA1 region. The drug induces pyramidal cell degeneration, loss of neuronal fibers, and activation of the NLRP3-ASC-caspase-1 inflammasome pathway, indicating its involvement in neuroinflammation and cell death. Spatial memory is impaired after two weeks of MA treatment, while recognition memory remains unaffected. Fear response memory is impaired after three weeks. These findings suggest that methamphetamine, even at lower doses, may have neurotoxic effects that impact memory and learning. However, further research is needed to fully understand its effects on other cognitive domains and inflammation pathways.