1. Background
Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) has presented a formidable worldwide health crisis in recent times. Since its first detection in 2019, the virus has swiftly traversed different continents. This has led to unparalleled efforts aimed at comprehending its clinical expressions and formulating successful approaches to mitigate its spread (1, 2). The virus leads to symptoms such as fever, cough, and shortness of breath. However, mounting evidence suggests that SARS-CoV-2 impacts various organ systems, resulting in a wide spectrum of symptomatic displays. Furthermore, muscle pain, headaches, and gastrointestinal symptoms have become common complaints, contributing to the complexity of the clinical portrayal of the illness (3, 4).
The global coronavirus disease 2019 (COVID-19) pandemic has highlighted the significance of understanding how viral infections affect at-risk groups, particularly individuals dealing with pre-existing health problems. One such group involves Parkinson's disease (PD), a neurodegenerative disorder marked by both physical and non-physical symptoms (5) (Figure 1).
Parkinson's disease has attracted attention due to its potential link to the severity of COVID-19 (6, 7). The age-related decline in the immune system and the potential immunomodulatory effects of certain Parkinson's medications could increase susceptibility to viral infections like COVID-19 (8).
Understanding the progression of COVID-19 is crucial, particularly given the diverse range of symptoms and the heightened risk faced by certain groups, such as individuals with PD (9). Despite extensive research on COVID-19, there remains a significant gap in our understanding of how the virus affects PD patients, particularly concerning neurological symptoms.
2. Objectives
This study aims to address this knowledge gap by comprehensively assessing laboratory indicators, clinical presentations, and post-infection outcomes in PD patients with active SARS-CoV-2 infections. By elucidating the specific neurological manifestations and their impact on PD progression, we aim to provide critical insights that can inform clinical management strategies and improve outcomes for this vulnerable population.
3. Methods
3.1. Data Collection
Data collection took place at 12 hospitals supervised by the country's viral diseases research network, which included Ali Asghar, Amin, Amiral Momenin, Imam Khomeini, Hajar, Khansari, Razi, Rouhani, Shariati, Sina, Vali Asr, and Yas Medical Center, between March 2020 and May 2021. Consent letters were obtained from each patient who participated in the study, which was approved by the Ethics Committee of Tehran University of Medical Sciences, Iran (IR.TUMS.VCR.REC.1399.599).
3.2. Study Participants
A total of 27 PD individuals with COVID-19 participated in the study, consisting of 9 women and 18 men, all of whom had previously been diagnosed with PD. The control group, on the other hand, comprised individuals diagnosed with PD but ruled out for SARS-CoV-2. All relevant information from patients' records was extracted. All patients referred to the corresponding hospitals with respiratory symptoms were suspected of SARS-CoV-2 infection during the peak of the pandemic. Patients with other underlying conditions such as diabetes, cancer, or any other co-infection with viruses and bacteria were ruled out from the study. Participants were excluded if they presented any underlying conditions that might complicate the interpretation of COVID-19 symptoms among PD patients, such as respiratory disorders, cardiovascular diseases, immunocompromised states, recent viral infections, or non-PD neurological disorders. The exclusion criteria underwent a comprehensive review process, meticulously assessing each potential participant's medical history, including comorbidities and recent illnesses. This rigorous evaluation ensured consistent and appropriate application of the exclusion criteria.
3.3. RT-PCR
Trained personnel collected nasal, oropharyngeal, or nasopharyngeal swabs following Iranian CDC guidelines. After extraction using the ROJE RNA extraction kit (ROJE, Iran), RT-PCR assays were performed in regional labs as recommended. All participants met the Iranian CDC criteria for COVID-19 diagnosis (10).
3.4. Statistical Analysis
Both SPSS version 27 and R version 4.2.3 were utilized for analysis. SPSS was employed for descriptive statistics, frequencies, ratios, the Chi-square test, and Fisher's exact test.
4. Results
A total of 54 Parkinson's patients were investigated in this study. Of these, 21 individuals (38.9%) were female, while 33 (61.1%) were male. Twenty-seven Parkinson's patients with positive COVID-19 tests were recruited as the case group, and 27 COVID-19 negative individuals were selected as the control group. Both the case and control groups were similar in age distribution (Mann-Whitney U test statistic = 331.0, P-value = 0.557). The first two columns of Table 1 show the frequency and percentage of each symptom in the case and control groups.
| Symptom | COVID-19 | OR | P-Value | 95% CI for OR | ||
|---|---|---|---|---|---|---|
| Positive (Case) | Negative (Control) | Lower | Upper | |||
| Cough | 22 (81%) | 23 (85%) | 0.77 | 0.715 | 0.18 | 3.23 |
| Rhinorrhea | 5 (19%) | 3 (11%) | 1.82 | 0.448 | 0.39 | 8.51 |
| Sore throat | 20 (74%) | 12 (44%) | 3.57 | 0.030 a | 1.13 | 11.25 |
| Shortness of breath | 22 (81%) | 7 (26%) | 12.57 | < 0.001 a | 3.43 | 46.02 |
| Diarrhea | 5 (19%) | 3 (11%) | 1.82 | 0.448 | 0.39 | 8.51 |
| Vomit | 3 (11%) | 1 (4%) | 3.25 | 0.321 | 0.32 | 33.41 |
| Fever | 24 (89%) | 15 (56%) | 6.40 | 0.010 a | 1.55 | 26.48 |
| Headache | 20 (74%) | 7 (26%) | 8.16 | < 0.001 a | 2.42 | 27.58 |
| Dizziness | 18 (67%) | 5 (19%) | 8.80 | < 0.001 a | 2.50 | 30.97 |
| Lethargy | 23 (85%) | 20 (74%) | 2.01 | 0.316 | 0.51 | 7.89 |
| Malaise | 21 (78%) | 19 (70%) | 1.47 | 0.536 | 0.43 | 5.03 |
| Muscle pain | 14 (52%) | 13 (48%) | 1.16 | 0.786 | 0.40 | 3.37 |
Frequency and Odds Ratio of Coronavirus Disease 2019 Symptoms in Case and Control Groups (Control Groups, n = 27)
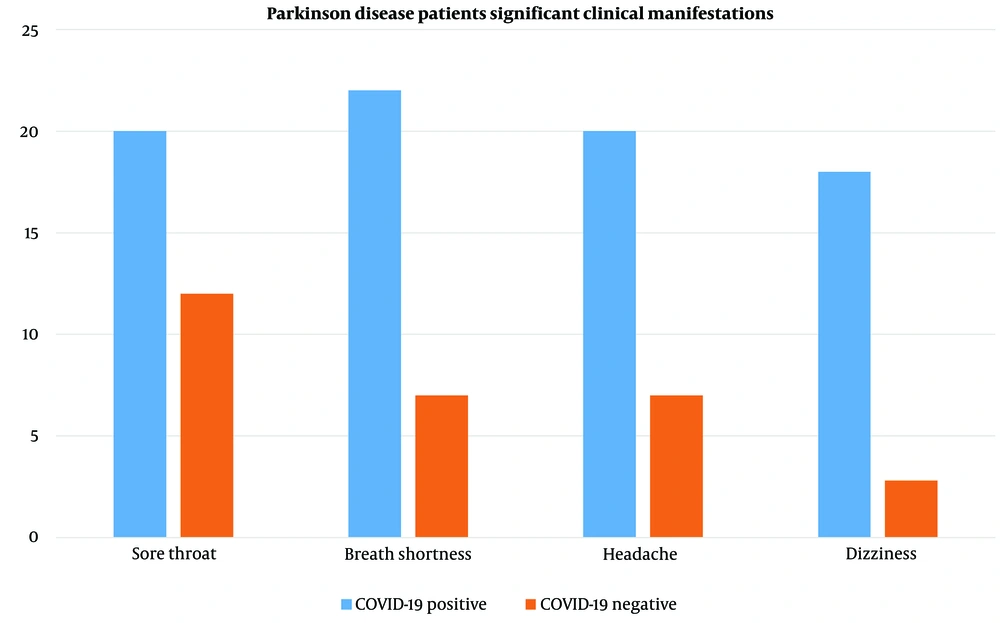
The odds ratio for each symptom in patients in the case group compared to the control group is also reported in Table 1. According to our data, the odds ratio of having a sore throat in the case group was 3.6 times higher than in the control group, which was statistically significant (P-value = 0.030). The odds ratio of experiencing shortness of breath in the case group was 12.6 times higher than in the control group (P-value < 0.001). Patients in the case group suffered from vomiting more than those in the control group. The odds ratio of having vomiting in the case group was 3 times higher than in the control group, though this was not statistically significant (P-value = 0.321). The odds ratio of having a fever in the case group was 6.4 times higher than in the control group (P-value = 0.010). Patients in the case group suffered more from headaches and dizziness than those in the control group. The odds ratio was 8.1 for headaches and 8.8 for dizziness (both P-values < 0.001). It should be noted that some confidence intervals are relatively large. Among the investigated symptoms, vomiting and rhinorrhea were lower in both the case (11% - 19%, respectively) and control groups (4% - 11%, respectively). In case group patients, there was an increased occurrence of headaches (74%) and dizziness (67%) (Figure 2).
To investigate further, a subgroup analysis was performed by sex. Table 2 shows the results for subgroup analysis and contains the frequency (percent) of patients who had each COVID-19 symptom and the odds ratio of each symptom in the case and control groups, separately for each sex. However, since the sample size in each group is relatively small and groups are rather unbalanced, some confidence intervals are extremely wide. According to the results in Table 1, having a sore throat in the case group was statistically higher than in the control group. In the female subgroup, the odds ratio of having a sore throat in the case group was 7 times higher than in the control group. However, the difference was not statistically significant, and the confidence interval is relatively wide, possibly due to the small sample size and unbalanced groups. In Table 2, fever was significant only in the female subgroup, while headache and muscle pain were significant only in the male subgroup. Dizziness was significant in both subgroups.
| Symptom | COVID-19 | OR | P-Value | 95% CI for OR | ||
|---|---|---|---|---|---|---|
| Positive (Case) | Negative (Control) | Lower | Upper | |||
| Cough | ||||||
| Female | 8 (87%) | 10 (83%) | 1.60 | 0.720 | 0.12 | 20.99 |
| Male | 14 (78%) | 13 (87%) | 0.54 | 0.514 | 0.08 | 3.45 |
| Rhinorrhea | ||||||
| Female | 2 (22%) | 2 (17%) | 1.43 | 0.749 | 0.16 | 12.70 |
| Male | 3 (17%) | 1 (7%) | 2.80 | 0.396 | 0.26 | 30.18 |
| Sore throat | ||||||
| Female | 7 (78%) | 4 (33%) | 7.00 | 0.054 | 0.97 | 50.57 |
| Male | 13 (72%) | 8 (53%) | 2.27 | 0.265 | 0.53 | 9.67 |
| Shortness of breath | ||||||
| Female | 7 (78%) | 3 (25%) | 10.50 | 0.024 a | 1.36 | 81.05 |
| Male | 15 (83%) | 4 (27%) | 13.75 | 0.002 a | 2.55 | 74.30 |
| Diarrhea | ||||||
| Female | 2 (22%) | 0 | 0.83 b | 0.169 | 0.35 | 198.09 |
| Male | 3 (17%) | 3 (20%) | 0.80 | 0.805 | 0.14 | 4.70 |
| Vomit | ||||||
| Female | 1 (11%) | 0 | 4.41 b | 0.271 | 0.16 | 121.68 |
| Male | 2 (11%) | 1 (7%) | 1.75 | 0.662 | 0.14 | 21.43 |
| Fever | ||||||
| Female | 8 (89%) | 5 (42%) | 11.2 | 0.046 a | 1.04 | 120.36 |
| Male | 16 (89%) | 10 (67%) | 4.00 | 0.136 | 0.65 | 24.69 |
| Headache | ||||||
| Female | 6 (67%) | 3 (25%) | 6.00 | 0.065 | 0.89 | 40.31 |
| Male | 14 (78%) | 4 (27%) | 9.63 | 0.005 a | 1.95 | 47.44 |
| Dizziness | ||||||
| Female | 8 (89%) | 2 (17%) | 40.00 | 0.005 a | 3.05 | 524.83 |
| Male | 10 (56%) | 3 (20%) | 5.00 | 0.045 a | 1.04 | 24.03 |
| Lethargy | ||||||
| Female | 7 (78%) | 12 (100%) | 0.12 b | 0.169 | 0.01 | 2.85 |
| Male | 16 (89%) | 8 (53%) | 7.00 | 0.033 a | 1.17 | 41.76 |
| Malaise | ||||||
| Female | 8 (89%) | 12 (100%) | 0.23 b | 0.271 | 0.01 | 6.25 |
| Male | 13 (72%) | 7 (47%) | 2.97 | 0.140 | 0.70 | 12.63 |
| Muscle pain | ||||||
| Female | 5 (56%) | 12 (100%) | 0.05 b | 0.064 | 0.002 | 1.07 |
| Male | 9 (50%) | 1 (7%) | 14.00 | 0.020 a | 1.51 | 130.10 |
Frequency and Odds Ratio of Coronavirus Disease 2019 Symptoms in Case and Control Groups in Each Subgroup
5. Discussion
The significance of Parkinson's disease and COVID-19 is profound (11). Meanwhile, the COVID-19 pandemic underscores the urgency of infectious disease management and robust public health strategies (2). Consistent with the findings of prior research (12, 13), our study also confirmed a higher prevalence of neurological symptoms, specifically headaches, dizziness, and fever.
A recent study conducted in Iran provided insights into the prevalence of neurological symptoms in SARS-CoV-2 patients. The most commonly reported neurological symptoms included headaches, disturbances in sleep patterns, hyposmia/anosmia (46%), and dizziness (45.4%). Incidence rates of headaches and dizziness in our population were higher than those recorded in this study (14). Additionally, another study conducted in the USA sought to understand how COVID-19 manifests in PD patients. It highlighted certain percentages among respondents to the COVID-19 symptom questions. Weakness was reported in 20 cases, while dizziness and confusion were reported in 13 and 11 cases, respectively. This research demonstrates the significant impact of neurological manifestations in individuals with COVID-19 and emphasizes the need for a comprehensive understanding of the diverse clinical manifestations associated with the virus (15).
Parkinson's disease is caused by the loss of neurons, impacting dopamine production, a key neurotransmitter (16). Severe acute respiratory syndrome coronavirus-2 triggers a strong immune reaction, marked by significant inflammatory substances, which may potentially impact dopamine neurotransmission (17). It has been suggested that neuroinflammation in COVID-19 patients could disrupt dopaminergic regulation, potentially contributing to Parkinson's disease, as cytokines may decrease vesicular monoamine transporter 2 (VMAT2), responsible for dopamine uptake and storage (18, 19). Moreover, COVID-19 infection may downregulate ACE2 receptors, potentially decreasing Dopa decarboxylase (DDC) expression, a crucial element for dopamine synthesis (20, 21). Therefore, in cases where Parkinson's disease and COVID-19 coincide, patients may experience worsening previously existing neurological symptoms in 58.8% of instances and severe COVID-19 in 11.7% of cases (22).
Similar to our results, headaches and dizziness as neurological indicators were prevalent clinical manifestations in PD patients following COVID-19. Additionally, a study conducted in Mexico City showed that shortness of breath (86.1%), fever (83.6%), and cough (77.8%) were the most prevalent symptoms in COVID-19 patients. Among the neurological symptoms, headache had the highest severity at 41.7% (23). Headaches accompanying SARS-CoV-2 infection may be attributed to direct SARS-CoV-2 damage, inflammation, low oxygen levels (hypoxemia), coagulation abnormalities, and endothelial damage (24).
In another research study, a remarkable prevalence and expression of neurological symptoms were noted in COVID-19 patients with mental disorders. The study revealed substantial occurrences, with headaches reported in 71.4% of individuals with schizophrenia, 50% of those with bipolar disorder (BD), 66.7% of migraine sufferers, and 83.3% of individuals with Alzheimer's (25). Psychiatric conditions such as schizophrenia and bipolar disorder exhibit chronic inflammation with augmented levels of IL-1β, IL-6, TNF-α, and IFN-γ, potentially enhancing susceptibility to SARS-CoV-2 infection due to immune dysregulation (26). Moreover, environmental stress due to the SARS-CoV-2 pandemic has heightened psychological strain worldwide, which could exacerbate existing psychiatric conditions (27).
In the context of respiratory symptoms, there are various studies to discuss. Similar to our study, in which fever (88.9%) and cough (81.5%) were common symptoms, a study conducted in Wuhan, China in 2019 reported fever (88.7%) and cough (67.8%) as the most common symptoms (28). The presence of cough in individuals with PD can be attributed to various factors such as dysphagia, impaired airway clearance, and altered respiratory muscle control. When combined with the respiratory implications of COVID-19 infection, these factors have the potential to worsen the occurrence of cough symptoms in PD patients (29). Parkinson's disease cases could be at heightened risk of severity of COVID-19 due to respiratory muscle stiffness, weakened cough reflexes, and existing breathing difficulties (30). Conversely, another study diverges from our findings, indicating a lower prevalence of shortness of breath (31%) and sore throat (5%) (31). A study conducted in China in 2020 reported the prevalence of shortness of breath and sore throat among patients as 31.2% and 17.4%, respectively (32). Furthermore, aligning with prior research, another study highlighted fever, cough, and shortness of breath as common symptoms of COVID-19, with frequencies of 98%, 76%, and 55%, respectively (33). Additionally, a study on the clinical symptoms of COVID-19 in the Iranian population reported cough in 94% of infected individuals. Rhinorrhea was experienced by 61 out of 94 respondents, accounting for 65% of the sample, and a sore throat was reported in 72% of infected individuals, which is close to our result (3).
After analyzing gender sub-groups between the case group (PD patients with positive COVID-19) and the control group (PD patients without COVID-19), we found that fever was significant only in the female subgroup, while headache and muscle pain were significant only in males. Dizziness was significant in both subgroups. However, large confidence intervals indicate that the inferences should be used with caution. In contrast to our findings, a study involving 60,648 community-dwelling adults revealed that among those testing positive for COVID-19, men exhibited a higher prevalence of fever (22.6% vs. 17.1%) and chills (14.9% vs. 12.6%) compared to women (34). Moreover, another study found that female patients with COVID-19 experienced higher rates of headache and fatigue (35).
In managing Parkinson's disease during the COVID-19 pandemic, interventions focusing on mood stability are crucial for patients with a psychiatric history affected by COVID-19 stress (11). When treating COVID-19 in Parkinson's patients, it's essential to monitor for drug-drug interactions. Combining monoamine oxidase inhibitors (MAOIs) used for PD with cough suppressants like dextromethorphan for COVID-19 symptoms can lead to serious interactions, potentially causing serotonin syndrome due to amplified serotonergic effects. Furthermore, concurrent administration of MAOIs and nasal decongestants can result in a severe hypertensive disorder (36).
In conclusion, our study has highlighted a significant observation: Parkinson's patients who contract COVID-19 are more likely to manifest neurological symptoms, underscoring the imperative of comprehensive care for this at-risk patient group. While we have explored the origins of these neurological symptoms, including underlying conditions and physiological aspects, it is essential to acknowledge that COVID-19 primarily presents with respiratory symptoms.
Despite having a statistically sound study population, we recommend future investigations with a larger sample size to enhance the robustness of our findings. Additionally, to minimize potential confounding factors, it is advisable to carry out the study on individuals without underlying medical conditions or considering their medication usage. Another constraint in our study was its retrospective nature, focusing on patients with concurrent COVID-19 and Parkinson's disease. Given the potential for prolonged or persistent neurological symptoms post-COVID infection, conducting a cohort study with patient follow-up could offer valuable insights.
Additionally, we found an increase in headaches among patients with both COVID-19 and Parkinson's disease. Since headaches are typical in COVID-19, we should interpret these findings carefully. This symptom may be solely due to COVID-19 rather than worsening neurological symptoms of PD. To better understand this, we should examine the previous headache history in Parkinson's patients and include brain imaging in future studies.