1. Background
The brain's capacity to store, maintain, and retrieve information is referred to as memory (1). Learning involves the neural mechanisms by which a person changes their behavior as a result of an experience, while memory refers to the storage mechanism of learned information (2). Physiologically, long-term memory is categorized into two types: explicit and conceptual (3). Explicit or declarative memory is accompanied by awareness and is created as a result of conscious effort (4). Explicit memory is necessary to perform activities. Conceptual or non-declarative memory is created as a result of direct experience and does not require conscious processes (5).
Memory disorders occur as a result of the destruction of neuroanatomical structures in the hippocampus, which prevents the storage, maintenance, and recall of memories (6). Memory disorders can be progressive, such as in Alzheimer's disease (AD), or temporary, like those caused by a blow to the head. Additionally, factors such as stress, pain (6), and stroke can lead to memory deterioration (7).
In chronic diseases where a person experiences pain for a long time, various psychological disorders can occur, affecting the processes of learning and memory. One such disease is ureteral obstruction. Several factors can cause unilateral or bilateral ureteral obstruction, including kidney stones and severe constipation, which mainly occurs in children (8).
Given the importance of enhancing the quality of life for individuals enduring chronic pain, such as from ureteral obstruction, and exploring the effect of pain on the hippocampal neuronal structure, this research was undertaken.
2. Objectives
Our objective is to quantify the number of neurons in the CA1 region and evaluate neuroinflammation in animal models of unilateral ureteral obstruction (UUO). Notably, the impact of pain and endorphins following ureteral obstruction on CA1 pyramidal cells has not been previously investigated.
3. Methods
In the current case-control study, 27 male Wistar rats weighing 230 - 280 g were obtained from the animal facility at Ilam University. They were acclimated to the new environment for one week in the animal house of Ilam University of Medical Sciences and randomly divided into three groups: (1) Sham group (n = 9), (2) UUO group (n = 9), and (3) UUO treated with naloxone IP injection (UUO + NA; n = 9). The study protocol was approved by the ethics committee of Ilam University of Medical Sciences under approval code: IR.MEDILAM.AEC.1401.003. All animals received appropriate care according to the criteria outlined in the Guide for the Care and Use of Laboratory Animals announced by National Institutes of Health.
3.1. Surgery
Unilateral ureteral obstruction, resulting in complete obstruction of the ureter, was performed under general anesthesia induced by using ketamine and xylazine (80/20 mg/kg). In the UUO group, the left renal ureter was separated from its surrounding tissues via an abdominal incision and then obstructed using two knots (0.4 suture thread) to ensure complete obstruction. The surgical site was then sutured using 0.4 thread. In the sham group, the surgical procedure was performed without ureteral obstruction. Rats in both the sham-operated and UUO groups were kept in the animal facility for five days post-surgery without further intervention.
In the case of the UUO + NA group, following anesthesia induction, left ureteral obstruction surgery was performed. Subsequently, the rats in this group received naloxone at a dose of 1 mg/kg body weight (i.p; 8 am) for five consecutive days post-surgery. Briefly, six days after ureteral obstruction surgery, five rats from each group were anesthetized using ketamine (150 mg/kg) and perfused transcardially with 4% paraformaldehyde in PBS solution. The rat brains were then fixed in paraformaldehyde solution and paraffinized.
3.2. Sectioning
Brain slices with a thickness of 20 µm were cut using a microtome three days later. All sections were placed on gelatin-covered slides, and tissue staining was done using the Nissl method.
3.3. Cresyl Violet Acetate Solution
To prepare a 0.1% solution, 0.1 g of cresyl violet was added to 100 mL of distilled water. A few drops of glacial acetic acid were added before use. The solution was then warmed and filtered for optimal results.
3.4. Nissl Staining Protocol
Initially, the tissue underwent deparaffinization with xylene (10 min, 2x) to eliminate embedding wax, followed by rehydration using 100% alcohol (5 min, 2x). This is followed by subsequent washes in 95% alcohol (3 min) and 70% alcohol (3 min), and then a rinse in distilled water (5 - 8 min). Staining was achieved with cresyl violet (2 - 5 min). Excess dye was then removed with distilled water and alcohol. Dehydration follows with 95% alcohol (2 - 8 min), 100% alcohol (5 min, 2x), and finally, xylene (5 min, 2x), preparing the tissue for mounting on slides.
3.5. Counting the Number of Neurons in the CA1 Area of the Hippocampus
Slices were collected from the areas between -3.3 to -4.5 behind the bregma. At least three slides were prepared from each rat, with three to five sections placed on each slide. For each section, three visual fields were observed under 40x magnification using an optical microscope.
3.6. Criteria for Neural Counting
Each neuron with an intact cell membrane and an obvious nucleus was counted as a healthy, preserved neuron and counted in the specified area.
3.7. Biochemistry Analysis
3.7.1. Sample Preparation
Six days after surgery, four rats in each group were anesthetized with ketamine (150 mg/kg) and xylazine (15 mg/kg). The skull was detached, and the brain tissue was immediately removed for brain homogenate preparation. TNF-α and nerve growth factor (NGF) levels were measured colorimetrically using the brain tissue homogenate. Samples were kept in a freezer at -80°C until use.
3.7.2. TNF Alpha
The TNF-α ELISA Kit (ab100785; USA) was used to quantitatively measure TNF-α in brain homogenate. We followed the manufacturer's protocol, which is briefly summarized here. The assay had a sensitivity of 25 pg/mL, with a detection range of 82.3 - 20,000 pg/mL.
Standards and samples were pipetted separately into the wells. After washing the wells, biotinylated anti-Rat TNF alpha antibody was added. HRP-conjugated streptavidin was then pipetted into the wells. The TMB substrate solution was added, and the color development, which was proportional to the amount of TNF-α, was measured at 450 nm.
3.7.3. NGF
The nerve growth factor ELISA kit (ABIN6958065; USA) was utilized to quantitatively detect NGF in rat brain homogenate. The assay had a detection range of 15.6 - 1000 pg/mL. Briefly, 100 μL of standards or samples were added to each well and incubated for an hour at 37°C. After incubation, 100 μL of detection reagent A was added and incubated at 37°C, followed by washing three times. This was followed by a 30-minute incubation with detection reagent B at 37°C. The wells were then incubated with the substrate for 15 minutes at 37°C, followed by the addition of the stop solution. Finally, the product was read at 450 nm immediately.
3.8. Statistical Analysis
The data were analyzed using Prism GraphPad (VI) software. Group comparisons were made using the Student's t-test followed by the Mann-Whitney post-test. The results were presented as mean ± SEM, with significance defined as P < 0.05 at a 95% confidence level.
4. Results
4.1. Unilateral Ureteral Obstruction Model
In UUO animals, the mean weight was 259.4 ± 4.36 g before surgery and changed to 230.2 ± 3.3 g at the end of the experiment. However, the mean weight in sham-operated animals was 256 ± 4.5 g before the experiments and changed to 265 ± 4.7 g at the end (approximately 11% weight loss in UUO animals).
4.2. The Average Number of Neurons in the CA1 Region
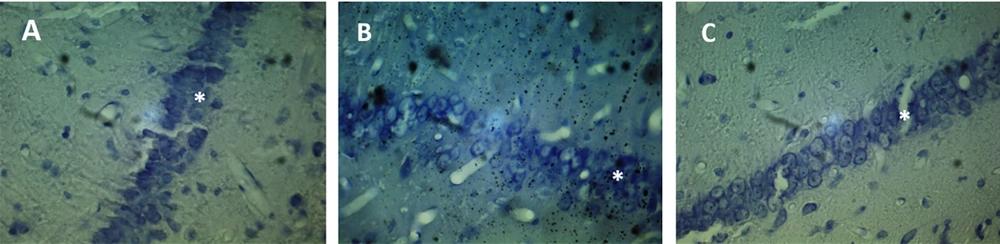
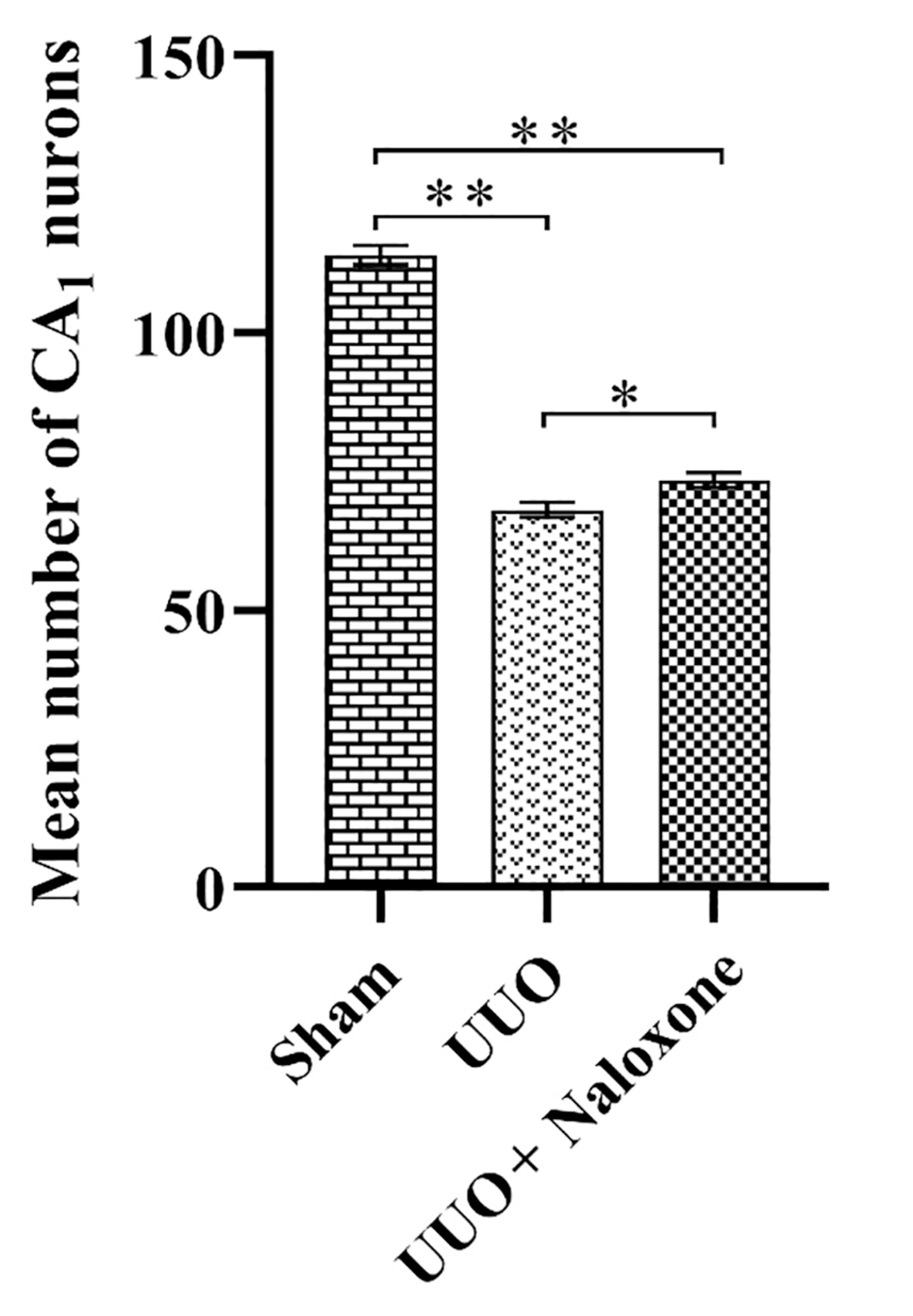
Brain slices were studied at distances between -3.3 and -4.5 behind the bregma, and the injection site is shown for the three groups in Figure 1. Neurons were counted in a blind manner. As shown in Figure 2, the mean number of neurons in the CA1 region was 68.11 ± 1.34 in the UUO group, 114.1 ± 1.77 in the sham-operated group, and 73.44 ± 1.37 in the UUO animals receiving naloxone.
The mean number of neurons in the CA1 region in UUO animals showed a significant decrease compared to the sham-operated group (P < 0.0001). Similarly, the mean number of neurons in the CA1 region in the UUO group receiving naloxone showed a significant decrease compared to the sham group animals (P < 0.0001). However, it is worth mentioning that the comparison of the mean number of neurons in the CA1 region in the UUO group receiving naloxone to the lesioned animals showed a significant increase (P < 0.0001).
The mean number of neurons counted in the CA1 region of the hippocampus in different groups. Sham group, unilateral ureteral obstruction group: UUO, unilateral ureteral obstruction animals receiving Naloxone: UUO + Naloxone. * UUO group vs. UUO + Naloxone group: P < 0.0001. ** Sham group vs. UUO group and UUO + Naloxone group: P < 0.0001.
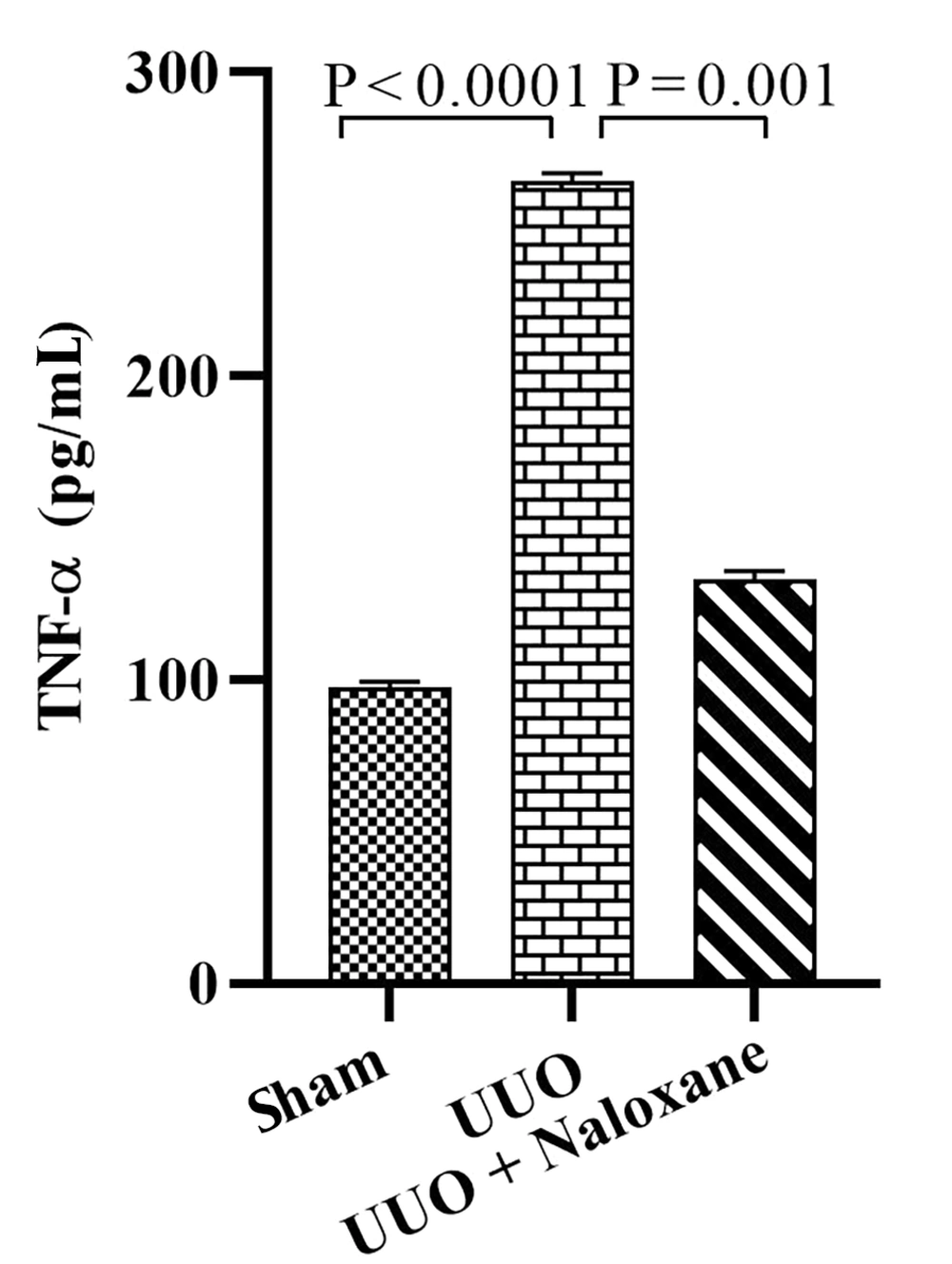
4.3. TNF-α
The TNF-α measurements are presented in Figure 3. In the sham group, TNF-α levels were 97.7 ± 1.7 pg/mL, while in the UUO group, this value was 264.3 ± 2.6 pg/mL, indicating a significant increase in the latter compared to the sham group (P < 0.0001). Furthermore, TNF-α levels were 133.17 ± 2.6 pg/mL in the UUO animals that received naloxone, showing a significant decrease compared to the UUO animals (P = 0.001).
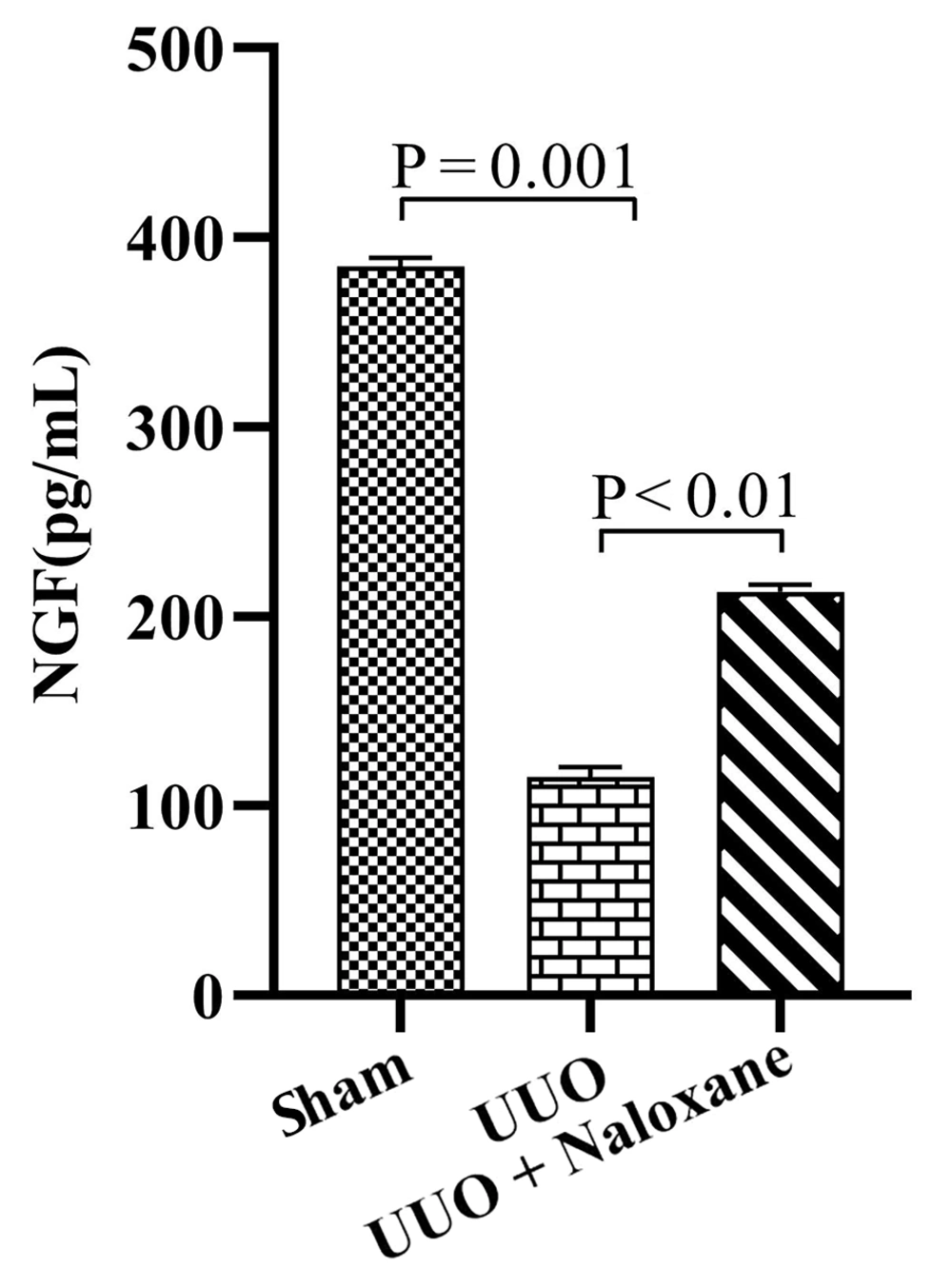
4.4. NGF
The level of NGF is shown in Figure 4. In the sham group, it was 384.8 ± 4.6 pg/mL, which significantly decreased in the UUO group to 115.2 ± 2.9 pg/mL (P = 0.001). However, the NGF level in the UUO + naloxone group was 213.1 ± 1.8 pg/mL, showing a significant increase compared to the UUO animals (P < 0.01).
5. Discussion
Our findings suggest that the UUO rat model could induce neurodegeneration in the hippocampus, while naloxone injection could ameliorate both neurodegeneration and inflammation in the animals. The hippocampus plays an important role in memory formation by providing a spatio-temporal framework in which the various sensory, emotional, and cognitive components of an experience are linked together (9).
The hippocampus is highly susceptible to damage from conditions such as epilepsy, hypoxia, ischemia, or encephalitis (10). Studies involving patients with hippocampal lesions have demonstrated that the main function of the hippocampus and its adjacent brain areas is to support the creation of new memories that can be transferred to conscious awareness (11). The signal transmission path for memory consolidation in the hippocampus extends from the entorhinal cortex to the dentate gyrus and continues to CA3 and CA1 (12). Therefore, damage to these areas of the hippocampus, as observed in AD, can affect learning and memory (13, 14).
Another factor that can influence the memory process is chronic pain. Research conducted in 2017 clearly stated that chronic pain can reduce cognitive ability and memory in affected individuals (15). Patients with chronic pain often complain of impairments in working memory and long-term memory (16). Chronic pain selectively affects memory processes that require more attention, such as working memory and recall in long-term memory (17).
The hippocampus and/or amygdala likely also play a role in chronic pain and are exposed to pain-induced plasticity (18). Animal models of pain are generated to mimic human pain experiences (19). Typically, small animals like mice and rats are utilized in these models. Acute pain is described as lasting for seconds to hours, while chronic pain persists for several days in these animal models (19, 20). In our study, we induced complete ureteral obstruction in the UUO animal model to replicate the intense abdominal pain or flank pain caused by different issues such as kidney stones (21). Since in UUO the pain persisted for several days, this model matches chronic pain. However, animals cannot communicate their pain experience like humans, leading to difficulties in assessing pain levels.
Clinical experience shows that there is an anatomical overlap between chronic pain and the learning and memory process (22). Expecting the imminent occurrence of pain activates the amygdala and hippocampus, and as soon as pain occurs, the activity of the amygdala decreases, but the activity of the hippocampus remains high (22-24).
In the present study, the mean number of neurons in the CA1 region in animals with UUO and chronic pain showed a significant decrease compared to the sham group. Additionally, other studies conducted by Schmidt in 2008 showed a reduction in grey matter in the amygdala region and several regions of the cerebral cortex in patients with chronic migraine compared to healthy individuals (25).
Furthermore, it has been reported that in patients with chronic pain, the volume of the bilateral hippocampus decreases significantly (26). All the mentioned studies are in line with the findings of the present research. Most studies on the relationship between chronic pain and memory processes have been conducted in non-visceral pain conditions such as neuropathic pain, migraine headaches, and chronic muscle pain (16, 27). In the present study, with the induction of the UUO model, chronic visceral pain was developed. The presence of pain in the UUO animal model is evidenced by symptoms such as weight loss, anorexia, and reduced social exploration in animals (28, 29). Our results showed anorexia and approximately 11% weight loss in animals undergoing UUO.
Pain stimulus increases pyramidal cell activity in the hippocampus and might be one of the mechanisms causing neurodegeneration in the CA1 region in chronic pain (30). The endogenous opioid system serves as the innate pain-relieving system and releases beta-endorphin, met-enkephalins, leu-enkephalins, and dynorphins (31). These opioids act via receptors known as mu, delta, and kappa. Like their endogenous counterparts, opioid drugs act on these receptors to produce analgesia or pain relief effects (32).
Naloxone hydrochloride, a synthetic N-allyl derivative of oxymorphone, functions as a pure opioid antagonist capable of blocking the effects of endogenous and exogenous opioids (33). It appears that naloxone exerts its antagonistic activity across all three opioid receptors. The effects of oral naloxone are initiated within 15 minutes and can last up to 24 hours (33). It has been reported that endorphins block inhibitory interneurons by binding to mu and delta receptors, increasing input to pyramidal cells, and enhancing their activity in the CA1 region (33).
Therefore, it seems that naloxone diminishes the increase in impulses entering the hippocampus by blocking the effect of endogenous endorphins at the hippocampal level, potentially inducing its neuroprotective effect in this manner (34). Moreover, endorphins at the spinal level reduce pain impulse signaling to higher levels, thus diminishing pain transmission (34). Consequently, endorphins may induce a dual effect in neuronal damage/protection following pain, yet their impact at the hippocampal level appears to lean towards neurodegeneration rather than neuroprotection (33), with naloxone exerting its neuroprotective effect through inhibiting this pathway. Nonetheless, naloxone may induce neuroprotective effects through alternative mechanisms as well (35, 36).
A study from 2021 revealed that naloxone has the capacity to diminish inflammatory responses by reducing the generation of inflammatory agents such as NO, TNF-α, IL-1β, IL-6, and COX-2, consistent with our recent investigation (37). Suppression of inflammatory pathways is crucial in averting neurodegeneration and modulating microglial activity across various brain regions. Naloxone achieves this effect by inhibiting ATP-dependent potassium channels (37).
Moreover, it has been observed that naloxone can protect the brain from neurotoxicity by reducing NOX activity and the production of reactive oxygen species (ROS) (35). Additionally, it exerts an antioxidative influence, counteracting the escalation of free radical production in the brain and mitigating cell death induced by apoptosis (35). Our current research aligns with prior studies exploring naloxone's protective effects on the brain, with the collective findings corroborating the results of our present investigation.
5.1. Conclusions
Our findings indicate that UUO induced neurodegeneration in the CA1 region of the hippocampus due to chronic pain in animals. The use of naloxone as an opioid antagonist immediately after UUO appeared to induce a neuroprotective effect in the CA1 region by blocking mu and delta receptors, inhibiting ATP-dependent potassium channels, scavenging free radicals, and reducing cytotoxicity.
Pain control in the context of human clinical conditions is crucial to prevent the possibility of neurodegeneration, which may persist as brain insults. This effect may be more pronounced in patients experiencing recurrent renal colic episodes. Therefore, it is essential to conduct human clinical studies and employ translational research methods alongside animal research to gain a better understanding of the exact mechanisms and outcomes of pain-induced neurodegeneration in the hippocampus.