1. Background
Multiple sclerosis (MS) is an autoimmune disease with the highest prevalence among nontraumatic neurological disabling disorders. The MS affects the central nervous system and is characterized by key features such as inflammation and demyelination of axons. It is a complex illness with an unknown underlying etiology. However, a combination of genetic factors and environmental influences—including exposure to ultraviolet B light, Epstein–Barr virus (EBV) infection, obesity, vitamin D deficiency, and smoking—appears to increase susceptibility to this neurodegenerative disease (1).
The MS is not confined to specific regions and poses a global health challenge, affecting both developed and developing countries (2, 3). A positive correlation has been observed between higher latitudes and greater prevalence. The highest rates are found in North America, Western Europe, and Australasia, while countries closer to the equator report the lowest rates (2). Additionally, there is a significant gender disparity, with females being more susceptible than males (4).
This neurodegenerative disorder typically manifests at a young age, with onset occurring between 20 and 30 years. It can lead to physical disability, cognitive impairment, reduced quality of life, and a shorter life expectancy (5).
The MS is diagnosed through a combination of radiographic findings, laboratory tests, and clinical signs and symptoms. Patients with MS commonly experience symptoms such as fatigue, pain, depression, ataxia, and cognitive impairment (6, 7). For instance, it is estimated that approximately 75% of MS patients suffer from cognitive dysfunction, while 95% experience fatigue (6). These symptoms often form a cluster in individuals with MS, which has a negative dose-response relationship with their quality of life (8). It is important to note that not all MS patients exhibit all these symptoms; some may experience only a subset.
Although MS is not curable, various pharmacological and non-pharmacological treatments are available to manage its symptoms. For example, an estimated 98 drugs are currently available for MS treatment, with more in development. However, the outcomes of medication-based therapies are often unsatisfactory, primarily due to potential serious side effects (9). As a result, attention has increasingly shifted toward more effective and safer non-pharmacological approaches with fewer complications, such as heat therapy, massage, ultrasound, and physical therapy (10, 11).
Neurorehabilitation is an innovative, noninvasive therapeutic modality characterized by its adaptability, affordability, safety, and user-friendly nature. These treatments aim to alleviate MS symptoms and improve the quality of life (12). Pulsed electromagnetic field (PEMF) therapy is a noninvasive neurorehabilitation approach widely used as an adjuvant to enhance symptom management in MS patients (13).
In PEMF therapy, intermittent pulses of current-generated magnetic fields are applied to living tissues over a brief period using a specific pulse repetition frequency. These magnetic fields induce an electrical current in conductive materials, resulting in secondary effects on the targeted tissues (14). It is hypothesized that alterations in axonal and synaptic neurotransmission may be one of the potential mechanisms underlying the action of PEMF (15).
In earlier research, Sandyk and Dann reported the therapeutic effects of PEMF on various MS symptoms through a series of case studies (16, 17). However, subsequent clinical trials produced mixed results. While some studies confirmed the effectiveness of PEMF (18, 19), others failed to observe significant improvements (20, 21).
2. Objectives
The primary objective of this research was to evaluate the impact of PEMF therapy on fatigue in individuals with MS, compared to a placebo. Additionally, the study sought to assess the influence of PEMF parameters on symptoms of depression and overall quality of life.
3. Method
This study was conducted following the guidelines of the preferred reporting items for systematic reviews and meta-analyses (PRISMA).
3.1. Search Strategies
A comprehensive search was performed using the PubMed/Medline, Scopus, and Web of Science databases to identify relevant studies published between 1990 and September 24, 2023. The keywords "multiple sclerosis" and "pulsed electromagnetic field therapy" were used in the search. Detailed search terms are provided in Appendix 1 in Supplementary File. The search was limited to studies published in English and Persian.
In addition to the mentioned databases, Google Scholar and the references of relevant studies were reviewed to ensure no studies were missed. If the full text of an article was unavailable, the corresponding author was contacted via email.
3.2. Criteria for Study Selection
The PICOS criteria were used to select studies, as follows:
(1) Population: Patients diagnosed with MS were eligible for inclusion. No restrictions were applied regarding age, gender, MS type, disease severity, or geographical location.
(2) Intervention: Trials examining the effects of PEMF therapy on MS patients were included.
(3) Comparison: Control groups included participants receiving placebo treatments, such as a magnetically inactive field or sham treatment.
(4) Outcomes: Symptoms such as fatigue, depression, and quality of life were considered primary outcomes.
(5) Study design: Clinical trials with parallel or cross-over designs were included.
Studies with other designs, as well as review articles, abstracts, conference reports, and book chapters were excluded.
3.3. Data Extraction
After completing the database search and manual review, all identified papers were imported into EndNote software. Two independent authors assessed the eligibility of the studies based on the inclusion and exclusion criteria. Subsequently, the authors independently extracted the following data: First author, publication year, participant characteristics, intervention protocol, duration of intervention, duration of study, and outcomes. This process was carried out by two authors (FR and SM). In cases of disagreement, issues were resolved by consensus or consultation with a third researcher (MB).
3.4. Risk of Bias Assessment
Two independent reviewers (NY and SM) evaluated the risk of bias, and a third reviewer (Ash) made the final decision in cases of conflict. The Cochrane risk of bias tool was employed to assess potential biases in the eligible studies (22). This instrument examines possible sources of bias across the following methodological domains: Random sequence generation, allocation concealment, reporting bias, performance bias, detection bias, attrition bias, and other potential sources of bias. According to the Cochrane handbook guidelines, the included studies were categorized as having a low risk (L), some concerns (S), or a high risk (H) of bias for each identified domain.
3.5. Data Analysis
The study results were systematically analyzed and described, with detailed findings presented in the text and Table 1 to highlight the characteristics and outcomes of the included studies. A meta-analysis could not be conducted due to the limited number of studies and participants, as well as the methodological, clinical, and statistical heterogeneity among the included studies.
| Study, Year | N (I/P) | Female, % | Age, y | Duration of Disease | Protocol for Control Group | Protocol for the Control Group | Outcome Measurements | |||
|---|---|---|---|---|---|---|---|---|---|---|
| PEMF Intensity | PEMF, % | Duration of Rehabilitation | Tool | Conclusion | ||||||
| Richards et al., 1997 (23) | 30 (15/15) | 70 | NA | NA | 5 - 10 µT | 4 - 13 Hz | Eight weeks; wearing a device: 10 - 24 h/day | Placebo device | 8-MSPS | Fatigue ↔ |
| Lappin et al., 2003 (19) | 117 | 76 | 21 - 64 | 1 - 13+ | NA | 1 - 25 Hz | Ten weeks (2-weeks interventions and 2 weeks washout); wearing a device 24 h/day | Placebo device | MSQLI | Fatigue ↓; quality of life↑ |
| Mostert andKesselring, 2005 (24) | 24 | 75 | 46.2 | NA | 17.5 myT | 50 Hz | Three - four weeks; 5 days/weeks; 2 sessions/day; 16 min/session | Sham therapy | FSS, VAS | Fatigue ↔ |
| Piatkowski et al., 2009 (18) | 37 (19/18) | I: 89.5; P: 72 | 45.75 | I: 10.5; P: 6.8 | 14 µT | NA | Twelve weeks; 2 sessions/day; 8 min/session | Sham therapy | MFIS, FSS , ADS-L | Fatigue ↓; depression ↔ |
| de Carvalho et al., 2012 (25) | 50 (25/25) | 70 | 46.75 | 15.2 | 37.5 µT | 4 - 7 Hz | Eight weeks; 3 sessions/weeks, 24 min/session | Sham exposures to magnetic fields | MFIS, FSS, VAS | Fatigue ↔ |
| Hochsprung et al., 2021 (21) | 24 (12/12) | 50 | 52.3 | 15.2 | 30 V. | 800 - 900 kHz | Three weeks; 5 sessions/weeks; 20 min/session | Sham treatment | MusiQoL, BDI-II, MFIS | Quality of life, depression, and fatigue ↔ |
| Bostani et al., 2022 (26) | 46 (23/23) | 52 | M: 34/4; F: 33.5 | NA | 4/5 mT | 15 Hz | Ten weeks; 2 sessions/weeks; 40 min/session | Placebo treatment | FSS | Fatigue ↔ |
| Granja-Dominguez et al., 2022 (20) | 44 (22/22) | 84.4 | 41 | 9.3 | 25 - 35 µT | 15 - 30 Hz | Four weeks; 5 sessions/weeks; 45 min per session | Magnetically inactive field | FSS; MFIS; BDI-II | Fatigue, depression, quality of life ↔ |
Abbreviations: N, number; I, intervention; P, placebo; PEMF, pulsed electromagnetic field; 8-MSPS, 8-item Multiple Sclerosis Performance Scale; MSQLI, MS Quality Of Life Inventory; FSS, Fatigue Severity Scale; VAS, Visual Analog Scale; MFIS, Modified Fatigue Impact Scale; ADS-L, a general depression scale–long version; MusiQoL, multiple sclerosis international quality of life; BDI-II, Beck Depression Inventory version II.
4. Results
4.1. Identification and Selection of Studies
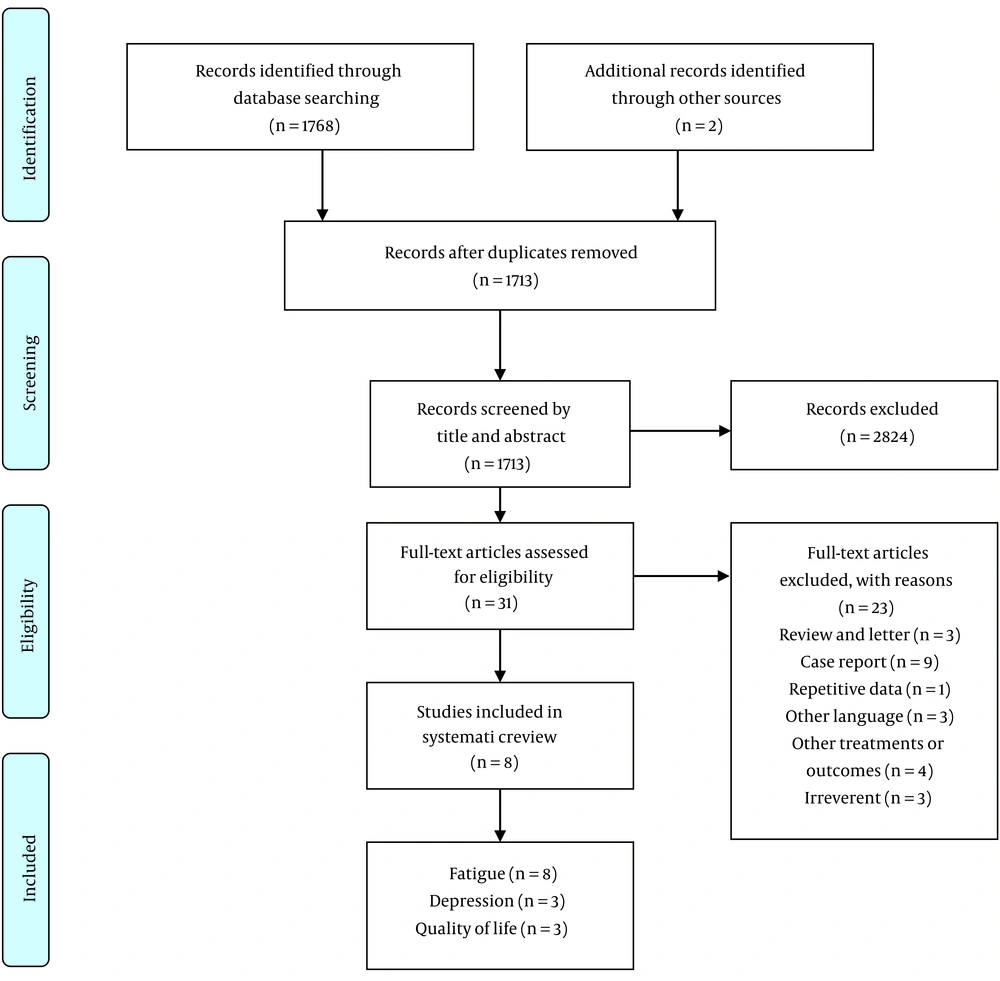
A total of 1,768 studies were identified from electronic databases, of which 57 were removed due to duplication. After screening titles and abstracts, 31 articles were selected for further assessment. Following a full-text review of these papers, only 8 met the inclusion criteria and were included in the review. The remaining studies were excluded for reasons including being case reports, reviews, observational studies, study protocols, involving other treatments, lack of access to full text, non-English or non-Persian language, and repetitive data. The flow diagram in Figure 1 illustrates the study selection process.
4.2. Description of the Included Studies
The overall population of the included studies consisted of 372 MS patients. All the studies were randomized clinical trials (RCTs), except for one, which was single-blind (26); the others were double-blinded. Additionally, two studies employed a cross-over design (19, 25), while the others used a parallel design. The duration of the interventions ranged from 3 to 12 weeks. In terms of participant gender, females constituted at least half of the participants, and all participants were over 21 years old.
4.3. Risk of Bias Results
The results of the risk of bias assessment are presented in Table 2. Based on these assessments, only one study was considered to have good quality, as all domains were rated as low risk (20). Five studies were classified as fair quality, as one or two domains were unclear (18, 19, 21, 24, 25). Finally, two studies were rated as poor quality, as they had a high risk in one domain and unclear ratings in multiple domains (23, 26).
| Study, Year | Sequence Generation | Allocation Concealment | Selective Outcome Reporting | Other Potential Threats to Validity | Blinding of Participants and Personnel | Blinding of Outcome Assessment | Incomplete Outcome Data |
|---|---|---|---|---|---|---|---|
| Richards et al., 1997 (23) | H | U | L | U | L | U | L |
| Lappin et al., 2003 (19) | L | L | L | U | L | U | L |
| Mostert and Kesselring, 2005 (24) | U | U | L | L | L | L | L |
| Piatkowski et al., 2009 (18) | U | L | L | L | L | L | L |
| de Carvalho et al., 2012 (25) | U | L | L | L | L | U | L |
| Hochsprung et al., 2021 (21) | L | U | L | L | L | U | L |
| Bostani et al., 2022 (26) | L | U | L | U | H | U | L |
| Granja-Dominguez et al., 2022 (20) | L | L | L | L | L | L | L |
Abbreviations: H, high risk of bias; L, low risk of bias; U, unknown risk of bias.
4.4. Outcomes of Interest
In this review, fatigue was considered the primary outcome, and it was reported in all 8 included studies. Fatigue was assessed using different tools, including the Modified Fatigue Impact Scale (MFIS) (18, 20, 21, 25), Fatigue Severity Scale (FSS) (18, 20, 24-26), Visual Analog Scale (VAS) (24, 25), the 8-item Multiple Sclerosis Performance Scale (8-MSPS) (23), and the Multiple Sclerosis Quality Of Life Inventory (MSQLI) (19). Of the eight studies, only two (18, 19) showed a significant reduction in fatigue levels in the PEMF group compared to the control group, while the remaining studies found no significant differences.
Several other variables, including quality of life and depression, were considered as secondary outcomes. Depression was measured in three studies using two tools: The Beck Depression Inventory-II (BDI-II) (20, 21) and the General Depression Scale — long version (ADS-L) (18). No significant improvement in depressive symptoms was observed in these studies. Quality of life was also evaluated in three studies using the MSQLI (19) and the multiple sclerosis international quality of life (MusiQoL) (20, 21). However, no improvement in quality of life was found following PEMF therapy.
5. Discussion
The aim of this study was to systematically examine the effects of PEMF therapy on fatigue, depression, and quality of life. Based on the results of this review, it appears that PEMF therapy is not superior to a placebo in improving these symptoms in MS patients; however, this result should be interpreted with caution.
Fatigue is a prevalent and disabling symptom that affects a significant number of individuals with MS, and it can have a substantial impact on their quality of life (27). As fatigue is inherently subjective, it is challenging to develop a universal tool to measure its level quantitatively (28). Consequently, fatigue severity is typically assessed using self-reported questionnaires, which can be influenced by disease severity as well as various social, environmental, and emotional factors (29). Approximately 30 questionnaires have been introduced to assess the level of fatigue, but the FSS and the MFIS are the most widely used in clinical trials worldwide (30). In the present review, five studies used the FSS, and four studies used the MFIS.
Since the etiology of fatigue remains unclear, finding an effective treatment is challenging. Therefore, the first line of treatment often involves addressing background factors that contribute to fatigue, such as pain, depression, and sleep disturbances (31). Additionally, several medications, including amantadine, paroxetine, modafinil, and 4-aminopyridine, can be used for pharmacological treatment. However, the efficacy of these treatments is inconsistent, and they may be associated with undesirable side effects (5). It seems that new treatments with less invasiveness are needed to improve this symptom. There are several complementary and alternative medicine approaches to improve MS symptoms, such as herbal medicine, physiotherapy, exercise therapy, reflexology, and magnetic field therapy (32).
Pulsed electromagnetic field therapy is a non-invasive and safe technique used as a complementary treatment for musculoskeletal disorders (33). For example, in a meta-analysis of fifteen clinical trials, PEMF was found to be effective in reducing pain in patients with osteoarthritis (34). Pulsed electromagnetic field is also used to improve the symptoms of several neurological disorders, such as stroke, Parkinson’s disease, Alzheimer’s disease, and MS (13). Several studies have assessed the effects of PEMF on MS symptoms, particularly fatigue, depression, and quality of life, though the results remain inconclusive.
In a trial conducted by Richards et al., the efficacy of PEMF therapy using an Enermed pulsing magnetic device in MS patients was evaluated using the Multiple Sclerosis Performance Scales (MSPS). After 8 weeks of treatment, patients in the active group showed a higher overall performance scale compared to the placebo group, although there was no difference in the fatigue subscale (23). Nevertheless, when the post-test was compared to the pre-test, the fatigue level showed a significant change only in the intervention group.
Furthermore, Lappin et al. conducted a multi-site, double-blind, placebo-controlled, crossover trial involving 117 participants treated with a small, portable PMFT generator. The results from the MSQLI tool indicated a significant improvement in fatigue levels and overall quality of life (19). In another study, Piatkowski et al. carried out a 12-week, double-blind, RCT to evaluate the anti-fatigue effects of a specific pattern of pulsed magnetic field therapy, bio electro-magnetic energy regulation (BEMER), on 37 MS patients. The fatigue level was significantly reduced in the treatment group compared to the sham therapy group, as measured by either the FSS or MFIS at the end of 12 weeks of intervention. However, fatigue scores did not change significantly after 6 weeks of treatment (18).
On the other hand, some studies did not show a significant difference between PEMF and placebo in terms of fatigue, although a slight positive effect was observed (20, 21, 24-26). In the study by Mostert and Kesselring., fatigue severity decreased by 18% in the intervention group, while it was only 7% in the control group; however, this difference was not statistically significant (24). Similar findings were reported in other studies (25, 26). Additionally, in the studies by Hochsprung et al. and Granja-Dominguez et al., when pre- and post-treatment results were compared, changes were noticeable only in the intervention group (20, 21).
Depression is another common symptom in individuals with MS, and its prevalence in this population is two to three times higher than in the general population (35). Depression plays a major role in determining the quality of life for MS patients (36). There is a complex relationship between depression, fatigue, and quality of life in patients with MS (37). Rodgers et al. proposed that fatigue could be an important mediator between depression and health-related quality of life; however, it can occur without depression (37). There is a lack of sufficient studies on the impact of PEMF therapy on depression and quality of life to draw a definitive conclusion (18-21). Of the three studies that evaluated the effects of PEMF on depression, none found positive effects, and only one study reported an improvement in quality of life (19). In contrast, some studies indicated that PEMF could have beneficial effects on depression and quality of life (38, 39). However, there were significant methodological differences between these studies. First, outcomes such as depression and quality of life were measured using different instruments. The populations in the present studies consisted of MS patients, who have a high rate of depression, but they were not diagnosed as clinically depressed individuals. In contrast, the studies by Larsen et al. (38) and van Belkum et al. (39) involved patients with treatment-resistant depression, where the severity of depression may have influenced the results. Additionally, the potential mechanisms of PEMF may be attributed to its effects on brain activity and connectivity. Since these effects depend on local applications, the studies mentioned used a transcranial method, while the included studies applied PEMF to the body.
It appears that various factors influence the effects of PEMF, with the duration of the intervention being one of them. Among the included studies, the one with the longest intervention (12 weeks) showed positive effects on fatigue (18). Based on biological reasoning, some evidence suggests that at least four weeks of treatment are needed to observe the effects of PEMF therapy (40). Therefore, the non-significant results of some studies could be attributed to the short duration of the intervention (21, 24). Additionally, although valid and reliable questionnaires were used in the included studies, there are some potential limitations in self-reporting assessments (41). Thus, it is recommended to use specific instruments designed for MS conditions or a combination of objective and subjective measurements (42).
Previous studies have shown that MS treatment is affected by different factors such as age, gender, and mental status (43, 44). Although the prevalence of MS is significantly higher among women than men, the prognosis of MS is worse in males. In other words, men tend to experience more severe and persistent symptoms, as well as faster deterioration of their neurological and cognitive functions, compared to women (45, 46). Furthermore, age is another confounding factor in MS patients, as people with MS exhibit different symptom patterns across various age groups (47, 48). In the included studies, women constituted the majority of the population, and the participants spanned a diverse range of age groups. Given the sex and age differences in MS severity, these factors may influence patient responses to treatment. Therefore, it may be beneficial to conduct targeted research on specific demographic subgroups of MS patients to provide valuable insights for optimizing therapeutic approaches.
The exact underlying mechanism of PEMF action has not yet been elucidated. Its analgesic, vasoactive, neurostimulatory, and trophic effects have been reported in some populations (49). It is hypothesized that PEMF could facilitate axonal conduction by altering calcium transport across the cellular membrane, which triggers a cascade of reactions leading to the synthesis of nitric oxide and other second messengers. This process subsequently results in cell regeneration and restores homeostasis (50-52). Other explanations include the release of melatonin from the pineal gland and neuroprotective effects through the modulation of inflammation and immune function (13, 15). The exposure time to PEMF, the duration of the intervention, as well as the intensity and frequency of PEMF, can influence outcomes.
Several limitations restrict the generalizability of this review’s findings. First, only 8 studies were included in the systematic review, and many of them had small sample sizes, which weakened the accuracy of the data and limited the power to detect treatment effects (53). Second, only publications in English and Persian were included, which may have led to missing some relevant data. Furthermore, although all studies used valid questionnaires to measure outcomes, some bias is inevitable due to the nature of self-reporting instruments. Finally, due to the high methodological heterogeneity and the small number of included studies, a meta-analysis was not feasible.
In conclusion, based on the results of the included studies, the effect of PEMF therapy on fatigue, depression, and quality of life in MS patients was minimal and, in many cases, no greater than sham treatment. Therefore, it cannot be recommended at this time as an approach to improve these symptoms. Nevertheless, this treatment may be useful for other symptoms or when applied with different therapeutic protocols. More clinical trials with longer follow-ups, larger sample sizes, advanced technological devices, and objective measurements are needed to determine the optimal frequency, intensity, intervention protocol, and duration of PEMF therapy.