1. Background
Stroke, the second leading cause of death globally and a major contributor to adult disability, exerts a profound toll on individuals and healthcare systems alike (1, 2). Chronic illnesses account for an astounding 80% of fatalities and 70% of years of life with a handicap each year, highlighting the urgency to tackle stroke's devastating impact (3). Despite advances in medical science, strokes continue to afflict over 15 million people worldwide each year, underscoring the pressing need for refined management strategies (4).
Physiologically, a stroke occurs when blood flow to the brain is obstructed, either by a clot in an ischemic stroke or by a rupture of a vessel in a hemorrhagic stroke (5). This interruption leads to rapid brain cell death due to lack of oxygen and nutrients (6, 7). Recognizing the signs of these events swiftly and implementing immediate treatment is crucial to minimize brain damage and improve outcomes (8).
The gravity of stroke's impact extends beyond immediate medical complications to encompass significant socio-economic repercussions (9). The substantial healthcare costs associated with acute stroke treatment and long-term rehabilitation impose a hefty burden on healthcare systems (10). Indirect costs related to loss of productivity and long-term disability further strain economies globally (11, 12). Therefore, addressing stroke's multifaceted consequences requires a holistic approach that integrates medical, economic, and social perspectives (13). This underscores the importance of effective prevention strategies, early diagnosis, and efficient management protocols to mitigate these extensive impacts.
In the realm of stroke care, identifying the intricate interplay of factors influencing in-hospital management is paramount (14, 15). By dissecting these variables and elucidating their roles, we can pinpoint modifiable factors that significantly impact stroke outcomes, paving the way for targeted interventions to reduce stroke-related mortality (14). Studies of this nature not only offer valuable insights into optimal patient care but also serve as benchmarks for the operational efficiency of stroke centers, guiding efforts to enhance overall stroke management practices (16).
Moreover, as the landscape of stroke treatment evolves with the advent of novel therapeutic modalities and management protocols, healthcare systems grapple with the challenge of ensuring their effective and widespread implementation (17-19). This necessitates a thorough evaluation of stroke care provision within the context of regional healthcare infrastructure, underscoring the importance of conducting studies within referral centers like Imam Reza Hospital in Tabriz.
Emergency Stroke Code (ESC) is a specialized protocol designed to expedite the management of acute stroke cases. Activation of the ESC triggers a coordinated response from emergency medical services (EMS) and hospital stroke teams, facilitating rapid assessment and initiation of appropriate interventions for stroke patients. By scrutinizing the effectiveness of pre-hospital interventions such as ESC code activation and intravenous thrombolytic therapy in patients with acute ischemic stroke, we aim to enrich our understanding of stroke care dynamics and inform targeted strategies to optimize stroke management within the region (20-22).
2. Objectives
In essence, this study seeks to bridge the gap between theoretical knowledge and practical application by translating insights gleaned from stroke care research into tangible improvements in patient outcomes. By leveraging the unique patient demographics and healthcare infrastructure of Imam Reza Hospital, we endeavor to advance the frontier of stroke management and bolster regional stroke care capabilities, ultimately contributing to the global effort to combat the burden of strokes on public health.
3. Methods and Result
In this cross-sectional study, all patients who referred to Imam Reza Hospital in Tabriz, a referral center for intravenous thrombolytic therapy, from April 2021 to March 2022 and had their ESC activated due to suspected stroke, or had received thrombolysis following the diagnosis of ischemic stroke were included.
Imam Reza Hospital holds a fundamental position as a referral center for the North-West region of Iran, catering to a diverse patient population from neighboring provinces. Given its strategic significance and substantial patient influx, conducting a comprehensive study within its precincts offers a unique opportunity to assess stroke management practices in a real-world setting.
Inclusion criteria were patients with activated ESC code or patients receiving thrombolysis with a diagnosis of ischemic stroke at Imam Reza Hospital. Patients with incomplete medical record information were excluded from the study.
Data was collected using ESC code information and data recorded in the Tabriz Stroke Registry. Collected data included demographic information of patients, stroke risk factors, time intervals between symptom onset and hospital admission, between hospital admission and brain CT scan, between hospital admission and thrombolysis injection, reasons for not administering thrombolysis, thrombolysis-related adverse events, mortality rate, outcomes, and 3-month follow-up of patients.
Data obtained in this study were analyzed using SPSS statistical analysis software version 16. Descriptive statistical methods were used to examine demographic variables, and results were presented as mean (standard deviation), frequency percentages, and frequency tables and charts. Quantitative variables were compared using the independent t-test, whereas qualitative variables were compared using the chi-Square test. P-values less than 0.05 were regarded as statistically significant.
Tabriz University of Medical Sciences Ethics Committee approved this study. All patient information was kept strictly confidential. Since no intervention was performed in this study and only patient follow-up results were extracted from the registry and clinical records, obtaining informed consent was not necessary.
3.1. Intravenous Thrombolysis Treatment
A total of 748 patients met the study's inclusion criteria, with 165 of them undergoing intravenous thrombolysis treatment. Among these, 117 patients had activated ESC, facilitating thrombolysis administration, while 48 patients did not benefit from ESC code activation but still received thrombolysis treatment (Table 1).
| Variables | tPA Not Administered | tPA Administered |
|---|---|---|
| Patients with ESC code activation | 583 | 117 |
| Patients without ESC code activation | 0 | 48 |
| Total | 583 | 165 |
Number of Patients Undergoing Intravenous Thrombolysis Treatment
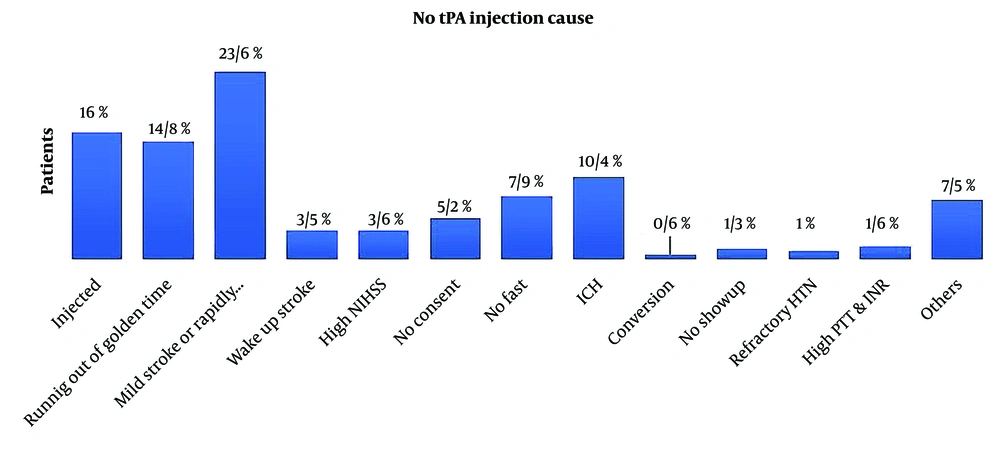
The main reasons for not administering tPA in patients with ESC code activation included mild symptoms or low National Institutes of Health Stroke Scale (NIHSS) scores (23.6%), exceeding the golden time for thrombolysis (14.8%), and hemorrhagic stroke (10.4%), as shown in Figure 1. Out of 694 patients with ESC code activation, 390 (56.2%) were related to the health monitoring system, while 238 (34.3%) were related to the emergency system (Figure 1).
3.2. Demographic Information and Stroke Risk Factors
The median age of patients receiving thrombolysis was 69 years, with a range from 59 to 77 years. Among the thrombolysis recipients, 58.2% were male. Hypertension was the most prevalent risk factor, affecting 70.3% of patients, followed by diabetes mellitus (23.6%) and high cholesterol (23.6%). Other risk factors included ischemic heart disease (17%), atrial fibrillation (9.1%), history of stroke (20%), and history of smoking (29.7%). When comparing patients with and without ESC code activation, the distribution of demographic characteristics and risk factors was generally similar between the two groups (Table 2).
| Variables | Total (N = 165) | tPA with ESC Code Activated (n = 117) | tPA Without ESC Code Activated (n = 48) |
|---|---|---|---|
| Age | 69 (59 - 77) | 68 (58 - 78) | 70 (59 - 76) |
| Gender (male) | 96 (58.2) | 66 (56.4) | 30 (62.5) |
| Hypertension | 116 (70.3) | 84 (71.8) | 32 (66.7) |
| Diabetes mellitus | 39 (23.6) | 26 (22.2) | 13 (27.1) |
| High cholesterol | 39 (23.6) | 27 (23.1) | 12 (25) |
| Ischemic heart disease | 28 (17) | 18 (15.4) | 10 (20.8) |
| Atrial fibrillation | 15 (9.1) | 11 (9.4) | 4 (8.3) |
| History of stroke | 33 (20) | 23 (19.7) | 10 (20.8) |
| History of smoking | 49 (29.7) | 37 (31.6) | 12 (25) |
Demographic Information and Stroke Risk Factors in Patients Undergoing Intravenous Thrombolysis a
3.3. Side Effects of Thrombolysis
The most common adverse event associated with thrombolysis was intracranial hemorrhage, occurring in 6.7% of patients overall. Among patients with ESC code activation, the incidence of intracranial hemorrhage was slightly lower at 6%, compared to 8.3% in patients without ESC code activation. Systemic bleeding was rare, occurring in only one patient with ESC code activation. Other side effects, such as allergic reactions or minor bleeding, were reported in 3% of patients overall, with similar proportions observed between patients with and without ESC code activation (Table 3).
| Variables | Total (N = 165) | tPA with ESC Code Activated (n = 117) | tPA Without ESC Code Activated (n = 48) |
|---|---|---|---|
| Intracranial hemorrhage | 11 (6.7) | 7 (6) | 4 (8.3) |
| Systemic bleeding | 1 (0.6) | 1 (0.9) | - |
| Other side effects | 5 (3) | 2 (1.7) | 3 (6.3) |
Side Effects of Intravenous Thrombolysis a
3.4. Comparison of Time Intervals and Clinical Outcomes
The time intervals between symptom onset and hospital arrival, hospital arrival and CT scan, and hospital arrival and tPA injection were significantly shorter in patients with ESC code activation compared to those without activation (P-Value < 0.001). Specifically, the median time from hospital arrival to tPA injection was 37 minutes for patients with ESC code activation, compared to 44 minutes for those without activation. However, there were no significant differences in NIHSS scores at admission and discharge, or in modified Rankin Scale (mRS) scores at discharge and 3-month follow-up between the two groups (Table 4).
| Variables | tPA with ESC Code Activated (n = 117) | tPA Without ESC Code Activated (n = 48) | P-Value |
|---|---|---|---|
| Time interval (minutes): Symptom onset to hospital arrival | 101 (67 - 161) | 95 (66 - 133) | 0.390 |
| Time interval (minutes): Hospital arrival to CT-scan | 13 (9 - 19) | 12 (9 - 24) | 0.640 |
| Time interval (minutes): Hospital arrival to tPA injection | 37 (29 - 37) | 44 (39 - 63) | < 0.001 |
| NIHSS at admission | 14 (9 - 19) | 15 (11 - 19) | 0.751 |
| NIHSS at discharge | 10 (3 - 16) | 10 (3 - 16) | 0.851 |
| mRS at discharge | 3 (2 - 4) | 3 (2 - 4) | 0.955 |
| mRS at 3-month follow-up | 3 (1 - 4) | 3 (0 - 6) | 0.987 |
Comparison of Time Intervals and Outcomes in Patients Undergoing Intravenous Thrombolysis a
4. Discussion
In this study, pre-hospital notification for patients with acute ischemic stroke using the ESC activation program demonstrated significant time savings in patient management, allowing physicians and patients more time for better evaluation and decision-making for this time-sensitive condition. Such close collaboration between hospitals and EMS systems enables citizens to benefit from a high-quality care network. Our study, like others in different countries, showed that pre-hospital notification for stroke patients improves in-hospital stroke care (23-25), resulting in a reduction in the time from hospital arrival to thrombolysis injection from 44 to 37 minutes.
It’s worth noting that the interval between symptom onset and arrival at the hospital was higher in patients receiving tPA in this study. If these patients had arrived at the hospital with the same timing as the non-TPA receiving group, the time from hospital arrival to thrombolysis injection would have been even less than 37 minutes. The greatest portion of the time interval between symptom onset and tPA injection is the time interval from symptom onset to hospital arrival, underscoring the importance of public education regarding the golden time of stroke to ensure patients contact the health system sooner.
Neurons in acute ischemic stroke die at a pace of 1.9 million per minute, making the condition very time-sensitive and highlighting the importance of rapid patient management (26). Patient outcomes have improved as a result of extensive initiatives, such as the Helsinki model, to eliminate delays in acute ischemic stroke therapy. The time for CT scanning, treatment decision-making, and thrombolytic drug administration in patients with acute ischemic stroke who were pre-notified by EMS improved (27, 28). Pre-hospital notification leading to shorter door-to-needle times was observed in our study. Some studies have also shown a significant reduction in door-to-needle time with pre-hospital notification (29-31), while others did not demonstrate the same effect (32, 33).
Small sample sizes or factors such as the initiation time of thrombolytic treatment being influenced by medical personnel, laboratory results, imaging tests, and communication between doctors, patients, or family members, as well as the speed of decision-making, could explain the disparities in the impact of pre-hospital communication on door-to-needle time in these studies. Weak or hesitant communication in decision-making can lead to treatment delays. These unmeasured confounding factors suggest that the relationship between pre-hospital communication and door-to-needle time may not be definitive.
Contrary to our expectations, there was no discernible difference in the clinical outcomes of the two groups; pre-hospital notification did not result in better clinical outcomes following IV tPA usage. The pre-informed group experienced a larger time interval between the onset of symptoms and hospital arrival than the other group, despite a dramatic reduction in DTN time in this group. This indicates that several problems exist in our system before reaching the hospital. Most likely, this delay in reaching the hospital in the pre-hospital communication group was due to longer transportation distances from our stroke center and the possibility of patients stopping at other hospitals, wasting time for most patients in this group.
In contrast, the majority of patients in the other group arrived at our hospital sooner since they were typically closer to the stroke center and didn't stop at another hospital en route. Prior research indicates that improved clinical outcomes following IV tPA administration have been significantly correlated with the interval between the onset of symptoms and treatment. Stated otherwise, the time curve indicates that a 20-minute delay in initiating therapy diminishes the likelihood of a positive outcome by over 20%, or around 1% every minute (34). Reducing DTN time did not, therefore, improve clinical outcomes in the pre-hospital communication group or shorten the time it took to get to the hospital.
Our study showed that a very small number of eligible patients for thrombolytic treatment (5.2%) refrained from treatment. This indicates that most patients accepted thrombolytic treatment despite the risk of intracerebral hemorrhage, following physician explanations about the benefits of treatment. This finding implies that emergency medical personnel should focus on recognizing stroke victims and administering the proper care.
Our investigation has certain shortcomings. First, the results of our study might not apply to all treatment facilities because it was carried out in a referral center that sees a high volume of patients from both the metropolitan area and neighboring regions. Second, the in-hospital protocols for treating stroke patients were standard across all participating hospitals. However, our study showed that when close collaboration is established between EMS systems and trained, organized hospitals, pre-hospital communication becomes a useful strategy. Another major limitation of the current study is the relatively low number of participants since we included data from only one year.
Additionally, there are limitations to using non-smart mobile phones for pre-hospital communication. Mobile phones only allow for single-person conversation, so EMS may have trouble contacting hospital employees. With this type of communication, hospital medical staff must contact all stroke team members separately after receiving EMS notifications. However, communication through smartphones enables all stroke team members in the hospital to be notified simultaneously via smartphone alerts and respond promptly. This enables various preparations to manage patients more rapidly. For instance, beds can be prepared for patients suffering from acute ischemic stroke prior to their initial evaluation, nurses can be ready to access venous catheters upon admission, and tissue plasminogen activator can be ready for injection at the time of patient admission. Neurologists can accompany patients through all investigations until a diagnosis is made, and radiologists can set up CT scan rooms for quick imaging of patients suffering from acute ischemic stroke. While pre-hospital notification is crucial for cutting down treatment time, so is the readiness of different medical professionals, including nurses, radiologists, neurologists, and emergency physicians, for patient admission.
4.1. Conclusions
Our study's findings have substantial implications for acute stroke intervention. Pre-hospital notification systems of this kind have the potential to boost tPA use and reduce in-hospital processing times. However, without a well-organized emergency infrastructure, including early diagnosis and effective dispatch systems, the previously described advantages may not enhance clinical outcomes following IV tPA for stroke patients. Therefore, while a pre-hospital communication system might help shorten the in-hospital processing time for IV tPA, more work needs to be done to improve effective out-of-hospital systems to enhance clinical outcomes following IV tPA.